Figure 5.
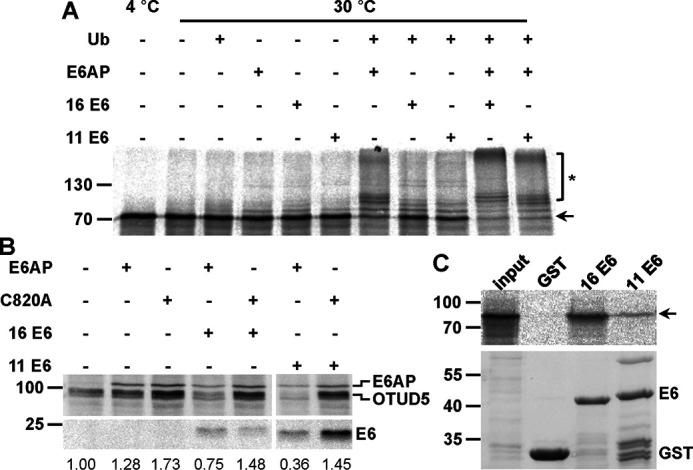
Validation of OTUD5 as a potential substrate protein of the HPV E6 proteins. A, in vitro translated, radiolabeled OTUD5 was incubated with E1 and UbcH7 in the absence and presence of ubiquitin (Ub), baculovirus-expressed E6AP, and GST fusion proteins of HPV-16 or HPV-11 E6 as indicated. Upon 90 min at 30 °C, reactions were stopped and analyzed by SDS-PAGE, followed by fluorography. Running positions of the nonmodified form and ubiquitinated forms of OTUD5 are indicated by an arrow and an asterisk, respectively. B, H1299 cells, in which endogenous E6AP expression is stably down-regulated by RNAi, were transfected with expression constructs encoding HA-tagged forms of OTUD5, E6AP, a catalytically inactive E6AP mutant (C820A), HPV-16, and HPV-11 E6 as indicated. 24 h after transfection, protein extracts were prepared and the levels of the various proteins were determined by Western blotting analysis using an anti-HA antibody. Note that prior to SDS-PAGE, the relative transfection efficiency of each transfection was determined (see “Experimental procedures”) to adjust the amounts of the extracts used for analysis. Relative levels of HA-OTUD5 are indicated. The analysis shown is representative of three independent experiments. Running positions of molecular mass markers are indicated. C, GST fusion proteins of HPV-16 E6 and HPV-11 E6 or GST alone bound to GSH Sepharose beads were mixed with in vitro translated, radiolabeled OTUD5. Upper panel, upon incubation for 90 min at 4 °C, beads were washed and eluates were analyzed by SDS-PAGE, followed by fluorography. input, 10% of OTUD5 used in the binding reactions. Lower panel, amount of GST proteins used in the binding reactions as determined by SDS-PAGE and Coomassie Blue staining.
