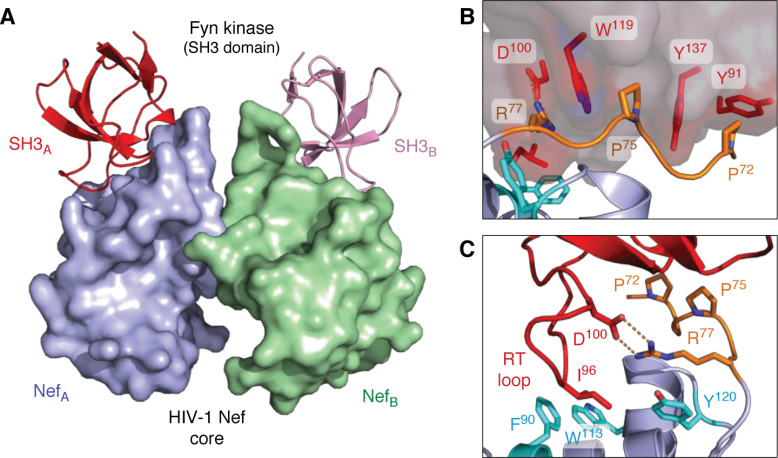
Figure 2.
X-ray crystal structures of HIV-1 Nef in complex with the SH3 domain of the Src-family kinase, Fyn. A, overall structure of the Nef core in complex with the Fyn SH3 R96I mutant. Nef·SH3 complexes form a 2:2 dimer, with Nef forming the dimer interface. The Nef monomers are modeled in blue (NefA) and green (NefB), respectively, with the SH3 domains in red (SH3A) and pink (SH3B). B, SH3 domain surface residues Tyr-91, Trp-119, and Tyr-137 form hydrophobic grooves that contact the Nef PxxPxR motif (orange). This interaction is oriented and stabilized by a polar contact between SH3 Asp-100 and Arg-77 from the Nef PxxPxR motif. C, high-affinity Nef·SH3 interaction requires Ile-96 from the SH3 RT loop (red), which accesses a hydrophobic pocket created by Nef residues Phe-90, Trp-113, and Tyr-120 (cyan). Structural details of the Nef homodimer interface from this complex are shown in Fig. 5. Models were produced with PyMOL using the crystal coordinates of the HIV-1 Nef core in complex with the Fyn R96I mutant SH3 domain (PDB code 1EFN).