Figure 5.
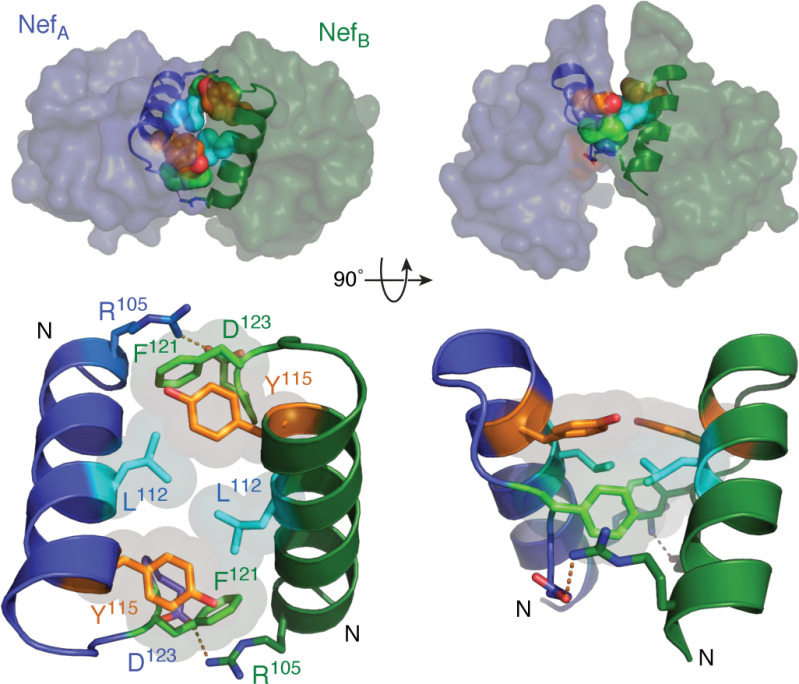
Nef homodimer interface from the X-ray crystal structure in complex with a Src-family kinase SH3 domain. Overview of the Nef dimer structure is shown in the top left, with the Nef monomers rendered in blue and green, respectively. The αB helices that form the dimer interface are highlighted (SH3 domains not shown for clarity). The αB helices are enlarged in the bottom left, illustrating the side chains of Leu-112, Tyr-115, and Phe-121 from each monomer, which form the hydrophobic core of the interface. Also shown are the reciprocal ionic contacts between Arg-105 and Asp-123. Both models are rotated 90° on the right. These models were produced with PyMOL using the crystal coordinates of the HIV-1 Nef core in complex with the Fyn SH3 domain R96I mutant (PDB code 1EFN). A model of the overall Nef·SH3 crystal structure is shown in Fig. 2A.