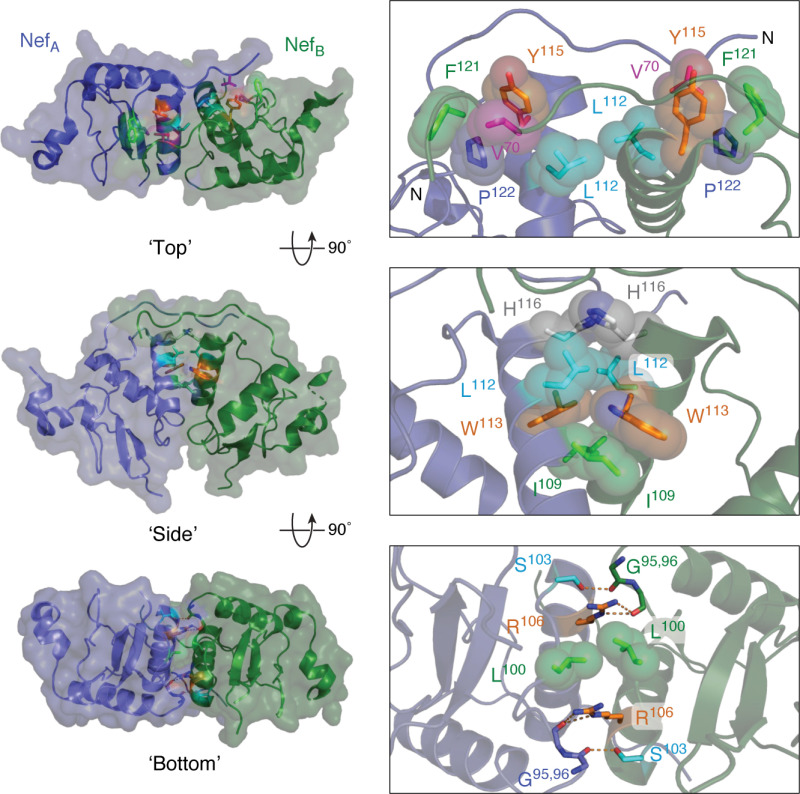
Figure 6.
Homodimer interface from the X-ray crystal structure of Nef in complex with the Hck SH3-SH2 regulatory region. Three views of this Nef homodimer are shown on the left with the Nef monomers rendered in blue and green, respectively (SH3-SH2 proteins not shown for clarity). Three interfaces stabilize this homodimer, which are enlarged on the right. In the top view, the side chains of Leu-112, Tyr-115, Phe-121, and Pro-122 form a hydrophobic cup that interacts with Val-70 from the opposing monomer in reciprocal fashion. The side view shows the contributions of Ile-109, Leu-112, Trp-113, and His-116 to a hydrophobic interface between the αB helices. The bottom view shows reciprocal polar contacts formed by Ser-103 and Arg-106 with the main-chain carbonyls of Gly-95 and Gly-96; Leu-100 also makes a nonpolar contact in this interface. These models were produced with PyMOL using the crystal coordinates of the HIV-1 Nef core in complex with the Hck SH3-SH2 region (PDB code 4U5W). A model of the overall Nef·SH3-SH2 crystal structure is shown in Fig. 3A.