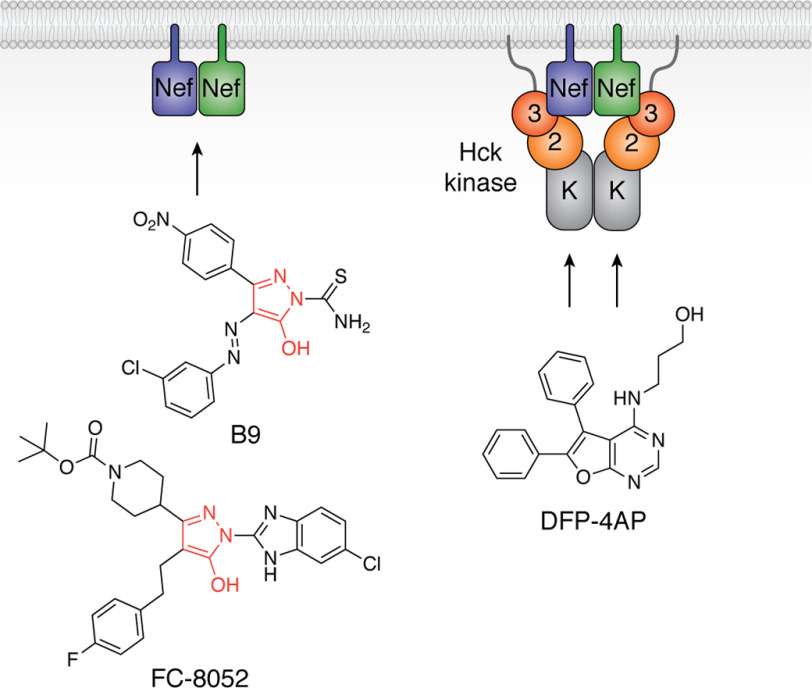
Figure 8.
Examples of small molecule inhibitors discovered using a Nef-coupled Hck kinase activity assay. Combining recombinant Nef and Hck proteins in vitro enabled high-throughput screening for inhibitors of the active complex (illustrated at the right; 3, SH3 domain; 2, SH2 domain; K, kinase domain). Screening of a small kinase inhibitor–biased library identified diphenylfuranopyrimidine 4-amino propanol (DFP-4AP), which inhibits the Nef-Hck complex via the Hck kinase domain (103). Screening of a large, more diverse library identified the compound B9 (102), which binds directly to Nef and inhibits Hck by an allosteric mechanism that may be related to Nef homodimer formation (left). Medicinal chemistry optimization has led to more potent analogs, such as FC-8052, which shares a hydroxypyrazole core with B9 (red). FC-8052 binds to recombinant Nef in vitro with a KD value of ∼10 pm, compared with about 80 nm for B9, and inhibits Nef-dependent HIV-1 replication in PBMCs in the subnanomolar range (100).