Abstract
Background:
With combination antiretroviral therapy (cART), infants with perinatally-acquired HIV (pHIV) are living into adolescence and adulthood. Worldwide, many have not received cART in the first years of life, and challenges of adolescence complicate transition to adulthood. Neurobehavioral outcomes in pHIV young adults (pHIVAd) are infrequently reported.
Objectives:
To examine neurobehavioral characteristics of pHIVAd ages 21 to 30, and to compare them with age-matched young adults infected in the second or third decade of life (HIVagematch), and older adults with similar duration HIV disease (HIVOA).
Methods:
A comprehensive neuropsychological test battery and questionnaires to determine cognitive function and mood, and reviews of neuromedical and behavioral records were undertaken in three groups of 13 individuals each. Descriptive analysis and bivariate techniques were used for comparisons.
Results:
Rates of cognitive impairment were highest in pHIVAd (85%) compared with HIVagematch (38%) and HIVOA (62%). pHIVAd had the worst scores in global cognition, speed of information processing, working memory, and verbal fluency (0.5 to 1.0 SD below other groups). There was a trend for higher rates of psychiatric dysfunction (predominantly mood disorders) in pHIVAd (85%) compared with HIVagematch (46%) and HIVOA (54%). Only 4 pHIVAd reported employment or enrollment in school. Four had autoimmune disorders.
Conclusions:
These pHIVAd displayed high rates of cognitive, psychiatric, and autoimmune dysfunction, greater than age-matched or HIV duration-matched comparators. While this small study is largely descriptive in nature, it suggests that a lack of cART in early life may result in long-term neurobehavioral and immune abnormalities manifesting into adulthood.
Introduction
It is estimated that in 2018, there were approximately 360,000 new HIV infections in children up to the age of 19, and 2.8 million children living with HIV worldwide (1). Approximately 90% of infected children were located in sub-Saharan Africa, with one quarter infected in the perinatal period (pHIV) through mother-to-child transmission (2). While availability of combination antiretroviral therapy (cART) has led to increased survival and enhanced testing and surveillance of infants born to HIV-infected mothers, in 2018 only 59% of 1,250,000 HIV exposed newborns were tested within the first 2 months of life (2). Thus, while current recommendations are for immediate therapy in infected neonates, it is often the case that there are delays in cART initiation, with current estimates that only 54% of the world’s infected children are receiving therapy (2,3). There is concern about long-term consequences of cART delay in infants and children, particularly with regard to cognitive/behavioral outcomes (4). The immature and rapidly developing nervous system is uniquely vulnerable to HIV in a variety of manners: as a function of direct CNS infection; via effects of severe systemic disease, immune dysregulation, opportunistic illness, and failure to thrive; and as a function of social and educational deprivation that may occur in resource limited settings.
Long-term consequences of delayed cART in pHIV are presently unclear, with indications that in some settings, acquired neurologic deficits may be resistant to remediation. In the Pediatric Randomized to Early versus Deferred Initiation in Cambodia and Thailand (PREDICT) trial, children provided immediate therapy at presentation between 1 and 12 years of age showed neurodevelopmental impairment after 144 weeks of therapy, similar to those with cART delayed until CDC stage C disease, and despite better immunovirologic outcomes; both groups performed significantly worse than HIV-negative children (5). In the Children with HIV Early Antiretroviral Therapy (CHER) trial, short-term motor and developmental outcomes were worse for infants with deferred therapy, when assessed at a median follow-up age of 11 months (6). Recent reviews describe the longer-term neurobehavioral outcomes of pHIV children and adolescents, with generally worse performance compared to HIV-uninfected and HIV-exposed uninfected (HEU) populations (7,8). A high degree of complexity in studying pHIV children and adolescents has been noted, as they experience variable time points for cART initiation, and unique social, developmental, and environmental challenges create impediments to continuity of care and medication adherence (7, 8, 9). Thus, ideal study design may be difficult to achieve as the variability of factors relevant to childhood development is large. With regard to CNS function, it is unclear how to predict a potentially wide range of trajectories in cognitive and behavioral maturation. In the face of these obstacles, 4 prior studies have extended neuropsychological observations into pHIV young adults (pHIVAd) up to the age of 26, demonstrating cognitive impairments relative to a variety of comparators, but with little information regarding other neurobehavioral phenomena (10–13).
In conducting a neuropsychological study, we recently encountered a group of pHIVAd born before the availability of cART, but experiencing the benefits of care in an urban medical center with specialized HIV programs and no significant resource limitations. All were provided access to epoch-appropriate standards of HIV care and ART as it became available. The neurobehavioral deficits observed in this group prompted us to compare their outcomes with age-matched young adults infected with HIV in their teens and twenties, as well as a group of older adults matched with the pHIVAd group for duration of HIV disease. Herein, we report these neurobehavioral observations.
Materials and Methods:
Participants:
The sample included 39 HIV-positive adults participating in one of two studies of CNS function at the Icahn School of Medicine at Mount Sinai (ISMMS): The Manhattan HIV Brain Bank (MHBB; U24MH100931), and a study examining the utility of a tablet-based application to detect neurocognitive impairment (R21NR015009). Both studies were conducted under oversight of the ISMMS Institutional Review Board, and all participants provided written informed consent, permitting review of the ISMMS medical record. The MHBB conducts a longitudinal cohort study as an information resource, with steady state population of 170; it is not hypothesis driven. The tablet application study enrolled 120 adults who allowed secondary use of collected data. Initially, 13 pHIVAd between the ages of 21 and 30 were identified in the tablet application study; then, a group of 13 age-matched individuals who acquired HIV in their teens and twenties (HIVagematch) were selected as comparators; then, a group of 13 older adults with HIV (HIVOA) were matched to pHIVAd on the basis of HIV disease duration, gender, and reading ability (determined with the Wide Range Achievement Test version 4), as these are predictors of neuropsychological test performance. The final sample ranged in age from 20 to 67 years, was fluent in English, and capable of undergoing a neuropsychological test battery.
Overall procedures:
As part of both studies, participants completed a comprehensive neuropsychological evaluation, and both interviews and medical record review were used to obtain neuromedical and psychiatric history, current medications including cART, and immunovirologic parameters, including plasma HIV RNA load and CD4 T-cell enumeration (for MHBB, these tests are run as routine study laboratories). Urine toxicology tests for amphetamines, barbiturates, benzodiazepines, buprenorphine, cannabis, cocaine, methadone, methamphetamine, opiates, oxycodone, phencyclidine, and tricyclic antidepressants were performed at the time of cognitive testing.
Neuropsychological assessment:
The neuropsychological test battery has been previously described (14, see supplemental table 1). Demographically and educationally-adjusted global and domain T scores were calculated for the following putative cognitive domains: motor function, processing speed, attention/working memory, memory encoding, memory retrieval, verbal fluency, and executive functioning. A modified version of the Lawton and Brody Activities of Daily Living Questionnaire (ADL) was used to assess functional dependence, defined by difficulty in at least 2 specified tasks (15). Cognitive symptoms were measured with the Patient’s Assessment of Own Functioning Inventory (PAOFI), a self-report measure of difficulties with memory, language and communication, sensory perceptual and motor skills, and higher-level cognitive functions, with impairment defined as 3 or more complaints (16). Neuropsychologic diagnoses were rendered on the basis of cognitive and functional information, using criteria specified in the research nosology for diagnosis of HIV-associated neurocognitive disorders (HAND) established in Frascati (17).
Psychiatric and neuromedical assessment:
The Beck Depression Inventory, second edition (BDI-II) was used to assess current depressive symptoms (18). The PAOFI was used to determine employment and educational status in pHIVAd and HIVagematch. Review of the medical record was used in both young adult groups to establish lifetime psychiatric and medical diagnoses, ART regimens and adherence, and immunovirologic status. For the older adult group, this information was elicited by a combination of medical record review and evaluation in the MHBB study.
Statistical analysis:
Groups were compared by ANOVA, logistic regression, Pearson’s chi square, or Wilcoxon/Kruskal-Wallis tests as appropriate, using Jmp version 9.0 and SPSS version 24. For infrequent attributes, descriptive analysis was employed.
Results
Demographic and immunovirologic characteristics (table 1):
Table 1.
Demographic and immunovirologic characteristics of the study population.
| pHIVAd | HIVagematch | HIVOA | F/χ2 | p | |
|---|---|---|---|---|---|
| Age in years (mean/SEM/range) | 24.7 (1.1)a Range 21-30 | 27.2 (1.1)b Range 20-32 | 56.6 (1.1)a,b Range 50-67 | 240.7 | <0.001 |
| Sex (% male) | 15%a | 92%a,b | 15%b | 21.2 | <0.001 |
| Ethnicity (% Hispanic*) | 31% | 38% | 54% | 1.5 | 0.476 |
| Race (%) | 9.0 | 0.173 | |||
| African American | 92% | 77% | 54% | ||
| Caucasian | 0% | 8% | 31% | ||
| Multiracial | 8% | 8% | 15% | ||
| Other/unreported | 0% | 8% | 0% | ||
| Years education (mean/SEM/range) | 13.1 (0.6)a Range 11-16 | 13.5 (0.6)b Range 8-16 | 10.1 (0.6)a,b Range 8-14 | 10.4 | <0.001 |
| Reading level (grade level; mean/SEM/range)** | 8.9 (0.7)a Range 4.2-13.0 | 11.8 (0.7)a,b Range 9.6-13.0 | 9.4 (0.7)b Range 4.2-13.0 | 4.5 | 0.018*** |
| Years with HIV (mean/SEM/range) | 24.7 (0.8)a | 4.8 (0.8)a,b | 24.0 (0.8)b | 208.5 | <0.001 |
| Age infected (mean/SEM/range) | 0.0 (1.3)a,b Range 0-0 | 22.5 (1.3)a,c Range 13-29 | 32.6 (1.3)b,c Range 23-45 | 167.9 | <0.001 |
| Current CD4 T cell count (cells/mm3; median/IQR) | 423 [177,555]a | 620 [486, 958]a | 632 [124, 709] | 6.4 | 0.040*** |
| Nadir CD4 T cell count (cells/mm3; median/IQR) | 118 [32, 155]a | 406 [252, 591]a,b | 100 [20, 244]b | 14.1 | <0.001 |
| Plasma HIV RNA load (log copies/ml; median/IQR) | 1.69 [1.28, 3.98] | 1.28 [1.28, 1.49] | 1.28 [1.28, 2.86] | 3.0 | 0.222 |
For each group, n=13. Within rows, means with the same superscript letter are significantly different at p<0.05. ANOVA with F ratio provided for age, years of education, reading grade level, years with HIV, and age at infection; Chi squares with Pearson χ2 provided for sex, ethnicity, and race; Wilcoxon/Kruskall-Wallis tests for current CD4, nadir CD4, and plasma HIV RNA load
Hispanic individuals endorsed African American (n=8), Caucasian (n=4), and multiple or other (n=4) race
Reading grade level was determined by the WRAT-4 reading test.
With Bonferroni correction for multiple comparisons, not significant
The three groups differed significantly in all characteristics except for race/ethnicity (over 90% of each group were minorities) and current plasma HIV RNA load (a majority were undetectable). HIVagematch had significantly more men, higher reading levels, fewer years with HIV infection, and higher CD4 nadirs than the other 2 groups. Only 4 people in the HIVagematch group had histories of CDC stage C disease, and cART formulations were available in the years during which they received initial HIV diagnoses (2007 through 2014). HIVOA were significantly older and had fewer years of education than the other groups; 10 HIVOA had histories of CDC stage C disease, and 11 received initial HIV diagnoses in years that cART was not available (1988 through 1997). pHIVAd had significantly lower current CD4 T cell counts than HIVagematch, and there was a hierarchy of ages of infection for the 3 groups (pHIVAd<HIVagematch<HIVOA); in the pHIVAd group, all but one individual had experienced CDC stage C disease, as all their birth years preceded cART availability (1985 through 1994). The single non-progressing pHIVAd individual appeared to be a virologic controller, with no documentation of CD4 counts below 900 cells/mm3. Everyone in the three groups was currently prescribed cART, and rates of current adherence (90% or greater fidelity to regimen) were: 92% pHIVAd, 83% HIVagematch, and 85% HIVOA. While they had a high rate of current cART adherence, only 38% of the pHIVAd group had lifetime adherence with prescribed regimens, which were offered as per epoch-appropriate standards of care.
Despite having worse current immunologic status (lower CD4) and higher historical rates of CDC stage C disease, the pHIVAd also had a relatively high frequency (31%) of diverse autoimmune/inflammatory phenomena, including: autoimmune hemolytic anemia, antiphospholipid antibody syndrome, systemic lupus erythematosus with protein S and C deficiencies, and Kawasaki disease. Similar autoimmune phenomena were not observed in the other two groups, although one HIVOA had a history of myositis and one HIVagematch carried a diagnosis of angioedema.
Neuropsychological characteristics (table 2, figure 1):
Table 2.
Neuropsychologic evaluation of the study population: rates of impairment and mean global and domain T scores (demographically corrected)
| pHIVAd | HIVagematch | HIVOA | F (df) / χ2 (df) | p | |
|---|---|---|---|---|---|
| Global cognition | 40.6 (1.5)a,b | 46.8 (1.5)a | 46.0 (1.5)b | 5.1 (2,36) | 0.011 |
| Motor | 41.6 (2.8) | 48.9 (2.4) | 49.2 (2.9) | 2.5 (2,29) | 0.104 |
| Speed of information Processing | 45.4 (1.9)a | 51.7 (1.9) | 52.3 (1.9)a | 3.9 (2,36) | 0.029 |
| Working Memory | 39.5 (2.1)a,b | 46.9 (2.1)a | 48.2 (2.1)b | 5.1 (2,36) | 0.011* |
| Memory encoding | 33.8 (2.8) | 37.1 (2.8) | 33.5 (2.8) | 0.5 (2,36) | 0.609 |
| Memory retrieval | 34.8 (2.8) | 41.1 (2.8) | 36.2 (2.8) | 1.4 (2,36) | 0.270 |
| Verbal fluency | 41.2 (3.1)a,b | 52.4 (3.1)a | 59.1 (3.2)b | 8.2 (2,35) | 0.001* |
| Abstraction/executive functioning | 45.1 (2.1) | 49.0 (2.1) | 47.3 (2.2) | 0.9 (2,35) | 0.428 |
| Percent cognitively impaired | 85%a | 38%a | 62% | 6.2 (2) | 0.046 |
Mean T scores in each domain (SEM); these were demographically and educationally adjusted as per (14). Within rows, means with the same superscript letter are significantly different at p<0.05; p determined by ANOVA (Bonferoni correction). Asterisks indicate cognitive domains that remain significant after applying an FDR correction (41) to control for multiple comparisons across 7 cognitive domains.
Percent cognitively impaired determined by Frascati criteria.
Figure 1.
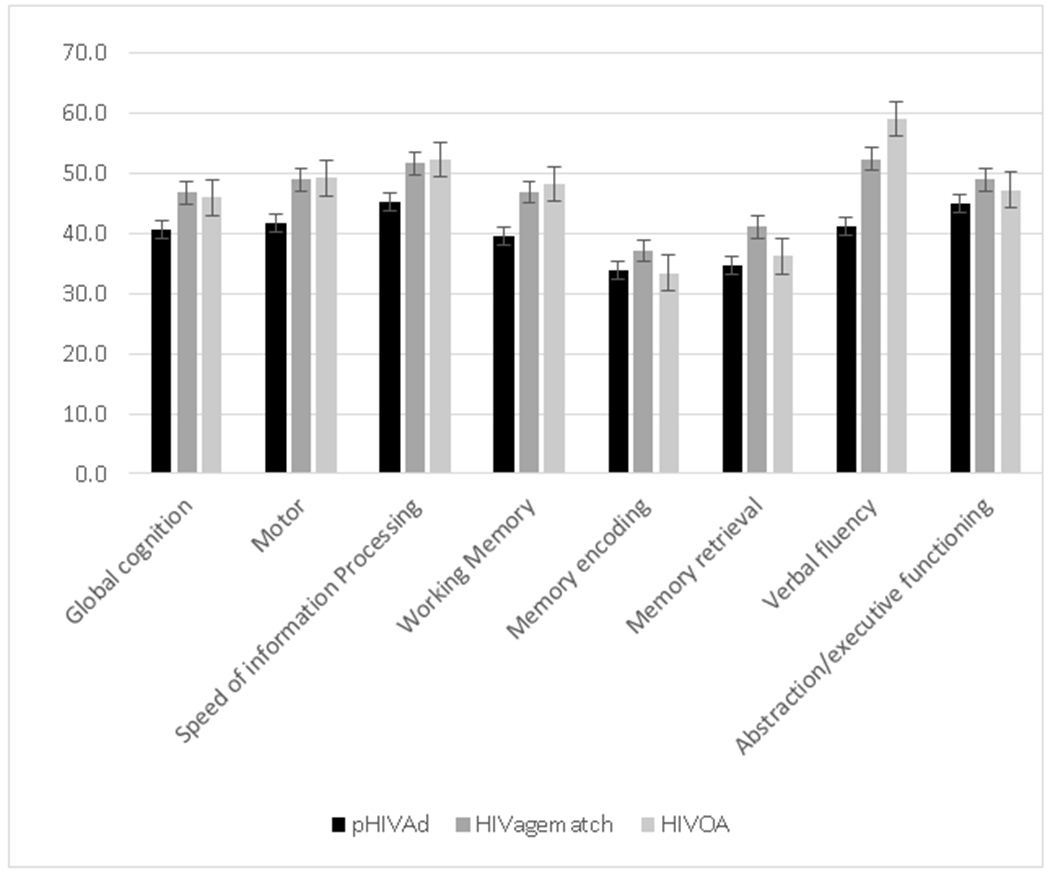
Mean global and domain T scores for neuropsychologic test battery
pHIVAd display significantly worse performance than other two groups in: global cognition, speed of information processing, working memory, and verbal fluency.
Error bar = standard error of the mean.
Standard deviations for domains: for global T score = 5.9; motor = 9.2; speed of information processing = 7.5; working memory = 8.3; memory encoding = 10.0; memory retrieval =10.3; verbal fluency = 13.2; abstraction/executive functioning = 7.6
pHIVAd: Perinatally-acquired HIV young adult group
HIVagematch: Young adults with recently acquired HIV (age-matched group)
HIVOA: Older adults with long duration of HIV disease (HIV disease-matched group)
Rates of cognitive impairment differed across the 3 groups (χ2=6.2, p=0.046), with pHIVAd exhibiting higher rates of impairment (85%) than HIVagematch (38%, p=0.023) and HIVOA (62%, p=0.197). pHIVAd had lower mean neuropsychologic test scores than other groups in the following domains: global cognition, speed of information processing, working memory, and verbal fluency. Significant group differences in working memory and verbal fluency remained after applying False Discovery Rate (FDR) corrections for multiple comparisons. The etiology for all impaired HIVagematch was thought to be HAND, whereas 64% of impaired pHIVAd were thought to have HAND and 36% to have other causes for cognitive abnormality (NPI-O; 3 had low pre-morbid reading ability, precluding assignment of deficits to HIV, and 1 had a remote cerebrovascular accident); in the HIVOA, 75% and 25% of impairments were HAND and NPI-O, respectively. On PAOFI, 77% of both pHIVAd and HIVOA, and 54% of HIVagematch, had 3 or more functional complaints, and 2 or more deficits in ADLs were endorsed by 8% of pHIVAd and HIVagematch, and 31% of HIVOA.
Neurobehavioral characteristics:
A wide range in depressive symptomatology was seen in all groups (scores ranging from 0 to 42 on the BDI), with mean scores of 13.5 in pHIVAd, 14.8 in HIVagematch, and 9.8 in HIVOA (F[2,36]=0.7, p=0.493). BDI scores of 14 or greater (the threshold for depression) were present in 38% pHIVAd, 31% HIVagematch, and 23% HIVOA. Psychiatric diagnoses were documented in 85% pHIVAd, 46% HIVagematch, and 54% HIVOA (2=4.9, p=0.086). For pHIVAd, diagnoses included: dysthymia and/or mood disorders (9 individuals); anxiety and panic disorders (2 individuals); bipolar disorder (1 individual), post-traumatic stress disorder (PTSD) (1 individual), attention deficit hyperactivity (1 individual), and oppositional defiance disorder (1 individual). For HIVagematch, 5 individuals had dysthymia and/or mood disorders, 2 had anxiety or panic, 1 bipolar, and 1 PTSD. One pHIVAd worked full time, 2 were employed part time, and 1 was a full-time student. In contrast, 4 HIVagematch worked full time, 4 were employed part time, and 2 coupled full time school with part time work. A high frequency of cannabis was present in urine toxicologies of both young adult groups, in 31% of pHIVAd and 38% of HIVagematch; illicit benzodiazepenes were also present in 2 pHIVAd.
Finally, adverse environmental factors were identified in many of the pHIVAd group. Histories of living in foster care, shelters, housing programs, or homelessness were ascertained in 38%, and histories of sexual assault or domestic violence in 54%; in combination, these adverse conditions were present in 69% of the sample. Histories of special education, being held back in school, and/or learning disabilities were present in 62%.
Discussion
Prior to the introduction of cART, 50% of all children born with HIV died before the age of two years (8). With cART-related declines in morbidity and mortality, many pHIV youth are now surviving into late adolescence and early adulthood (4). The impact of pHIV on children has been well researched, with some reviews describing generally worse neurodevelopmental outcomes in comparison to HEU and HIV-unexposed uninfected (HUU) children (19,20). This is not a universal phenomenon, and neurodevelopmental outcomes in pHIV treated within the first 3 months of life may be equivalent to HEU and better than pHIV with later therapy initiation (21). However, even if obstacles to early therapy initiation can be overcome, the unique aspects of maturing into adolescence with HIV present challenges that are not only medical, neurobiologic and behavioral, but also significantly influenced by environmental factors such as caregiver functionality, home and educational quality, and nutrition (8). The pHIVAd group described herein is unusual: uniformly born before availability of cART, yet surviving into adulthood despite significant social adversity. In part their survival may be attributable to the resource-rich medical environment in which they matured, where implementation of standards of care did not lag, and rehabilitative and supportive services were readily available. Thus, this group may in some manner represent an optimal medical outcome for pHIVAd who do not receive cART formulations in the first years of life. It is also of note that 11 of these 13 surviving pHIVAd were female. Recent observations document a strong bias towards female sex in elite control of pediatric HIV infection (22). Thus, it is possible that this group’s longevity represents a combination of standards of support and care, as well as innate biologic capacity to manage infection. Alternatively, it could reflect recruitment bias; in the New York City-based cART-era Child and Adolescent Self-Awareness and Health (CASAH) study, there is equal sex representation in pHIV adolescents and young adults recruited between the ages of 9 and 16 years, albeit it is unclear what percentage of the CASAH population was born prior to 1995 (13).
While this pHIVAd group demonstrated great longevity, it was not accompanied by advantageous cognitive outcomes. Compared to age-matched young adults with more recent acquisition of HIV and older adults with similar duration disease, they displayed increased rates of cognitive impairment and worse domain T scores in working memory and verbal fluency. This deficit distribution is consistent with prior studies of pHIV children; in one meta-analysis, most consistent domain impairments occurred in working memory, processing speed, and executive function (23–26). In one prior study of pHIVAd up to age 24 years, deficits in working memory, set-shifting/executive function, and motor speed were described (10). Impaired language skills have also been observed in pHIV children, with potential pathogenetic contributions of intrinsic (cognitive or hearing loss) and extrinsic (education, home environment) factors (8). Five of the pHIVAd reported herein had impairments in verbal fluency; 1 had documented hearing loss for which hearing aids were provided, 3 had significant stressors in the home environment, and 1 lacked recognizable contributing factors.
The etiology of diverse cognitive deficits in this sample is unclear, but likely related to both intrinsic vulnerability of the developing nervous system to medical and environmental adversity, as well as the severity of pre-cART systemic HIV disease. With regard to intrinsic vulnerability, CNS HIV compartmentalization has been demonstrated in a large percentage of young children naive to cART, and even without viral invasion of brain, systemic inflammation can have deleterious effects on neural development (27,28). Prior to 1995, it is likely that natural history HIV infection adversely affected the CNS of these pHIVAd, and similar to the findings of PREDICT, it appears that when available, cART did not result in cognitive remediation. History of CDC class C disease also shows strong associations with cognitive impairment in prior studies of pHIV, and in this pHIVAd group was almost universally present (20,29,30). This association is demonstrated by the AALPHI cohort: cognitive performance of pHIV adolescents without CDC class C disease was similar to HIV-negatives; those with prior CDC C diagnosis fared significantly worse in the domains of executive function, speed of information processing, and fine motor skills (31). The impact of severe illness may also transcend biology, as concomitant medical illnesses can result in prolonged absences from school, failure to complete learning assignments, and lack of socialization with peers. Social adversity, whether on the basis of medical illness or other environmental and social factors, has been related to cognitive outcomes in pHIV children and adolescents, with evidence that childhood adversity and HIV have combined effects on neural outcomes in adulthood (8, 32). Finally, repetitive and ongoing CNS insult cannot be ruled out, as less than half of the pHIVAd group maintained life-long ART adherence as documented in the medical record. The impediments to maintaining ART adherence through adolescence have been described, including pill fatigue, stigmatization, and concomitant psychiatric illness (33).
The pHIVAd group demonstrated high rates of psychiatric illness. Psychiatric dysfunction was also described in the CASAH cohort of pHIV adolescents, but in contrast to the predominance of mood disorders in our older pHIVAd group, anxiety disorders, which decreased with age, were most prevalent in CASAH (34, 35). There is evidence that childhood adversity is a strong risk factor for psychiatric symptoms and aberrant amygdala activity in older adults with HIV (36). While social stressors no doubt contributed to the persistent burden of mood disorders in our pHIVAd, a nascent literature suggests that early exposure to neuroinflammatory stimuli, through effects on microglial cell function, may impact neuronal circuitry and be associated with behavioral disorders emerging later in life (37, 38). Microglial cells have become central to the “multiple hit” hypothesis of psychiatric dysfunction, where behavioral symptoms are triggered by combinations of genetic predisposition and environmental stressors at critical stages of development (39). The participation of microglial cells in patterning neuronal networks, their vulnerability at developmental stages to stressors like HIV, coupled with decades of environmental stress as pHIV mature to young adults, may be contributing factors to the high rates of psychiatric disorders seen in these individuals.
Similar “patterning” effects on immunity are postulated to occur for immature immune systems stimulated at early ages, where a balance between pathogen-induced activation and self-tolerance occurs in the setting of unique T-cell and monocyte developmental stages (40). For example, neonatal monocytes, while typically in epigenetic conformations that suppress reactivity, may alter epigenetic silencing and undergo a prolonged anti-microbial response, with decreased levels of pathogen-induced cell death (41, 42). This could in theory result in a hyper-reactive state that persists and emerges in adulthood, as has been demonstrated in animal models (38). The current pHIVAd group had high rates of autoimmune disorders, a phenomenon not previously observed in descriptions of pHIV children. It could be speculated that this reflects the unique immunologic composition of a group surviving pHIV despite unavailability of cART in their birth years; alternatively, it may also be adult emergence of autoimmunity with early stimulation by HIV. It is also unclear whether heightened immunity influences cognitive development, as many models of neuropathogenesis entail negative effects of pro-inflammatory states. Future studies of children and young adults surviving with cART will be necessary to determine if our observation is unique or universal, and to determine its basis.
In this early description we recognize several factors that impact the applicability of our observations. First, this is a small sample fortuitously observed in the context of other studies focused on cognition and behavior, and its representativeness is uncertain. Sample size also limits the types of analyses that can be performed, but given the currently uncommon occurrence of pHIV between the ages of 21 and 30 years, descriptive analyses may still be valuable for understanding a unique population that will be increasing in the future. Second, we were unable to review un-interrupted medical records over a 30-year life-span, but relied on contemporaneous notes maintained in the electronic health record over roughly a decade. Third, an ideal comparator to assess HIV-specific effects on neurobehavior would have been HEU young adults, which were unavailable through our ongoing studies. Nevertheless, as there is a paucity of research on cognition and behavioral outcomes in pHIV infected young adults, we believe these preliminary observations are useful in examining outcomes in a resource-rich environment, as an indicator of the potential floor for burdens that may eventually arise in less advantageous environments. It will be important to have a thorough understanding of the effects of HIV and its treatment on the health and development of adolescents and young adults, to maximize their learning potential and overall functionality, and to plan for appropriate support systems as the world’s pHIV population ages.
Supplementary Material
References
- 1.United Nations International Children’s Emergency Fund, Global and regional trends July 2019. https://data.unicef.org/topic/hivaids/global-regional-trends/ accessed July 24, 2019
- 2.United Nations International Children’s Emergency Fund, Children. HIV and AIDS: Global Snapshot 2019. https://data.unicef.org/resources/children-hiv-aids-global-snapshot/ accessed July 24, 2019
- 3.US Department of Health and Human Services. Antiretroviral management of newborns with perinatal HIV exposure or perinatal HIV. https://aidsinfo.nih.gov/guidelines/html/3/perinatal/187/antiretroviral-management-of-newborns-with-perinatal-hiv-exposure-or-perinatal-hiv accessed July 24, 2019
- 4.Flynn PM, Abrams EJ. Growing up with perinatal HIV. AIDS 2019; 33:597–603. [DOI] [PubMed] [Google Scholar]
- 5.Puthanakit T, Ananworanich J, Vonthanak S, Kosalaraksa P, Hansudewechakul R, van der Lugt J, et al. Cognitive function and neurodevelopmental outcomes in HIV-infected children older than 1 year of age randomized to early versus deferred antiretroviral therapy: The PREDICT neurodevelopmental study. Pediatr Infect Dis J 2013; 32:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laughton B, Cornell M, Grove D, Kidd M, Springer PE, Dobbels E, et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS 2012; 26:1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellins CA, Malee KM. Understanding the mental health of youth living with perinatal HIV infection: lessons learned and current challenges. J Int AIDS Soc 2013; 16:18593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laughton B, Cornell M, Boivin M, VanRie A. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J Int AIDS Soc 2013; 16:18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nestadt DF, Lakhonpon S, Pardo G, Saisaengjan C, Gopalan P, Bunupuradah T, et al. A qualitative exploration of psychosocial challenges of perinatally HIV-infected adolescents and families in Bangkok, Thailand. Vulnerable Child Youth Stud 2018; 13(2):158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willen EJ, Cuadra A, Arheart KL, Post MJD, Govind V. Young adults perinatally infected with HIV perform more poorly on measures of executive functioning and motor speed than ethnically matched healthy controls. AIDS Care 2017; 29(3):387–393. [DOI] [PubMed] [Google Scholar]
- 11.Nagarajan R, Sarma MK, Thomas MA, Chang L, Natha U, Wright M, et al. Neuropsychological function and cerebral metabolites in HIV-infected youth. J Neuroimmune Pharmacol 2012; 7:981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paramesparan Y, Garvey LJ, Ashby J, Foster CJ, Fidler S, Winston A. High rates of asymptomatic neurocognitive impairment in vertically acquired HIV-1-infected adolescents surviving to adulthood. J Acquir Immune Defic Syndr 2010; 55(1):134–136. [DOI] [PubMed] [Google Scholar]
- 13.Robbins RN, Zimmerman R, Korich R, Raymond J, Dolezal C, Choi CJ, et al. Longitudinal trajectories of neurocognitive test performance among individuals with perinatal HIV-infection and -exposure: adolescence through young adulthood. AIDS Care 2019; doi: 10.1080/09540121.2019.1626343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol 2004; 26(6):759–778. [DOI] [PubMed] [Google Scholar]
- 15.Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsych Soc 2004; 10:317–331. [DOI] [PubMed] [Google Scholar]
- 16.Chelune GJ, Lehman RAW. Neuropsychological and personality correlates of patients’ complaints of disability In: Goldstein G, Tarter RE, editors. Advances in clinical neuropsychology. New York, NY: Plenum Press; 1986. pp. 95–118. [Google Scholar]
- 17.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck AT, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571. [DOI] [PubMed] [Google Scholar]
- 19.McHenry MS, McAteer CI, Oyungu E, McDonald BC, Bosma CB, Mpofu PB, et al. Neurodevelopment in young children born to HIV-infected mothers: A meta-analysis. Pediatrics 2018; 141(2):e20172888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowell CS, Malee KM, Yogev R, Muller WJ. Neurologic disease in HIV-infected children and the impact of combination antiretroviral therapy. Rev Med Virol 2014; 24:316–331. [DOI] [PubMed] [Google Scholar]
- 21.Jantarabenjakul W, Chonchaiya W, Puthanakit T, Theerawit T, Payapanon J, Sophonphan J, et al. Low risk of neurodevelopmental impairment among perinatally acquired HIV-infected preschool children who received early antiretroviral treatment in Thailand. J Int AIDS Soc 2019; 22:e25278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vieira VA, Zuidewind P, Muenchhoff M, Roider J, Millar J, Clapson M, et al. Strong sex bias in elite control of paediatric HIV infection. AIDS 2019; 33:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brakis-Cott E, Kang E, Dolezol C, Abrams EJ, Mellins CA. The impact of perinatal HIV infection on older school-aged children’s and adolescents’ receptive language and word recognition skills. AIDS Patient Care STDS 2009; 23(6): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen S, ter Stege JA, Geurtsen GT, Scherpbier HJ, Kuijpers TW, Reiss P, et al. Poorer cognitive performance in perinatally HIV-infected children versus healthy socioeconomically matched control. Clin Infect Dis 2015; 60(7):1111–1119. [DOI] [PubMed] [Google Scholar]
- 25.Koekkoek S, De Sonneville LM, Wolfs TF, Licht R, Geelen SP. Neurocognitive function profile in HIV-infected school-age children. Eur J Pediatr Neurol 2007; 12(4): 290–297. [DOI] [PubMed] [Google Scholar]
- 26.Phillips N, Amos T, Kuo C, Hoare J, Ipser J, Thomas KGF, et al. HIV-associated cognitive impairment in perinatally infected children: a meta-analysis. Pediatrics 2016; 138(5):pii: e20160893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sturdevant CB, Dow A, Jabara CB, Joseph SB, Schnell G, Takamune N, et al. Central nervous system compartmentalization of HIV-1 subtype C variants early and late in infection in young children. PLoS Pathog 2012; 8(12): e1003094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagberg H, Mallard C. Effect of inflammation on central nervous system development and vulnerability. Curr Opin Neurol 2005; 18:117–123. [DOI] [PubMed] [Google Scholar]
- 29.Smith R, Malee K, Leighty R, Brouwers P, Mellins C, Hittelman J, et al. Effects of perinatal HIV infection and associated risk factors on cognitive developmental among young children. Pediatrics 2005; 117(3):851–862. [DOI] [PubMed] [Google Scholar]
- 30.Smith R, Chernoff M, Williams PL, Malee KM, Sirois PA, Kammerer B, et al. Impact of human immunodeficiency virus severity on cognitive and adaptive functioning during childhood and adolescence. Pediatr Infect Dis J 2012; 31(6):592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Judd A, LePrevost M, Melvin D, Arenas-Pinto A, Parrott F, Winston A, et al. Cognitive function in young persons with and without perinatal HIV in the AALPHI cohort in England: Role of non-HIV-related factors. Clin Infect Dis 2016; 63(10):1380–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark US, Cohen RA, Sweet LH, Gongvatana A, Devlin KN, Hana GN, et al. Effects of HIV and early life stress on amygdala morphometry and neurocognitive function. J Int Neuropsychol Soc 2012; 18(4):657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prasitsuebsai W, Sethaputra C, Lumbiganon P, Hansudewechakul R, Chokephaibulkit K, Truong KH, et al. Adherence to antiretroviral therapy, stigma and behavioral risk factors in HIV-infected adolescents in Asia. AIDS Care 2018; 30(6):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mellins CA, Brackis-Cott E, Leu CS, Elkington KS, Dolezal C, Wiznia A, et al. Rates and types of psychiatric disorders in perinatally human immunodeficiency virus-infected youth and seroreverters. J Child Psychol Psychiatry 2009; 50(9):1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mellins CA, Elkington KS, Leu CS, Santamaria EK, Dolezal C, Wiznia A, et al. Prevalence and change in psychiatric disorders among perinatally HIV-infected and HIV-exposed youth. AIDS Care 2012; 24(8):953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark US, Sweet LH, Morgello S, Philip NS, Cohen RA. High early life stress and aberrant amygdala activity: risk factors for elevated neuropsychiatric symptoms in HIV+ adults. Brain Imaging Behav 2017; 11(3):649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thion MS, Ginhoux F, Garel S. Microglia and early brain development: an intimate journey. Science 2018; 362:185–189. [DOI] [PubMed] [Google Scholar]
- 38.Yockey LJ, Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity, 2018; 49:397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thion MS, Garel S. On place and time: microglia in embyronic and perinatal brain development. Curr Opin Neurobiol 2017; 47:121–130. [DOI] [PubMed] [Google Scholar]
- 40.Debock I, Flamand V. Unbalanced neonatal CD4+ T-cell immunity. Front Immunol 2014; 5:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bermick JR, Lambrecht NJ, denDekker AD, Kunkel SL, Lukacs NW, Hogaboam CM et al. Neonatal monocytes exhibit a unique histone modification landscape. Clin Epigenetics 2016; 8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dreschers S, Gille C, Haas M, Seubert F, Platen C, Orilikowsky TW. Reduced internalization of TNFa/TNFR1 down-regulates caspase dependent phagocytosis induced cell death (PICD) in neonatal monocytes. PLoS One 12(8): e0182415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A practical and powerful approach to multiple testing. J Royal Stat Soc Series B Stat Methodol 1995; 57(1):289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


