Figure 6.
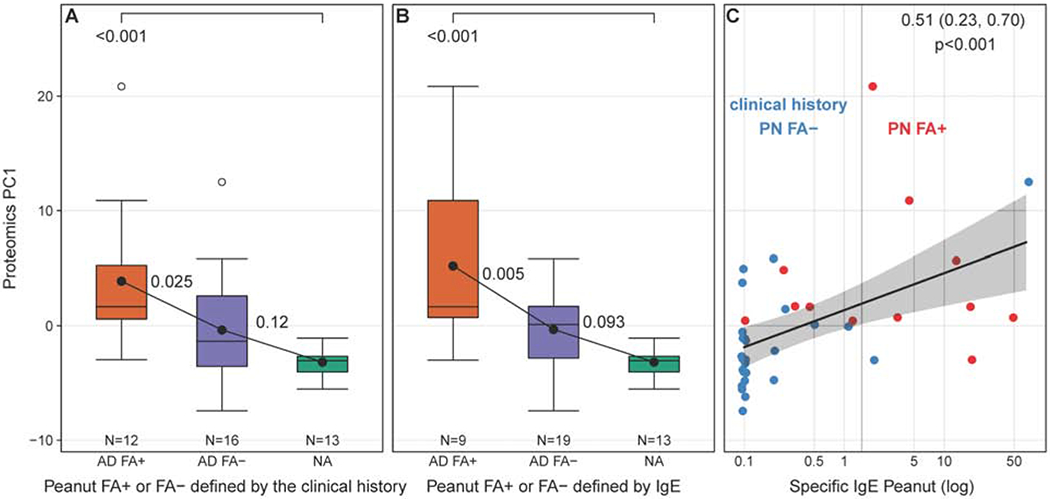
Validation of the PC1 results in STS analysis of non-lesional skin samples from the independent cohort of adults with AD with and without FA to peanut and NA controls. (A) The cumulative expression of PC1 proteins defined in the pediatric STS analysis was found to be differentially expressed in the proteomic analysis of STS from adults with AD and a history of clinical reactions to peanut, as compared to AD adults with no clinical history of allergic reaction to peanut and NA controls. (B) Expression of PC1 proteins was the highest in AD adults with the positive total IgE to peanut defined by the cutoff above 2kUA/L. (C) A significant positive correlation between total serum IgE levels to peanut and STS PC1 expresion was observed.
