Fig. 3.
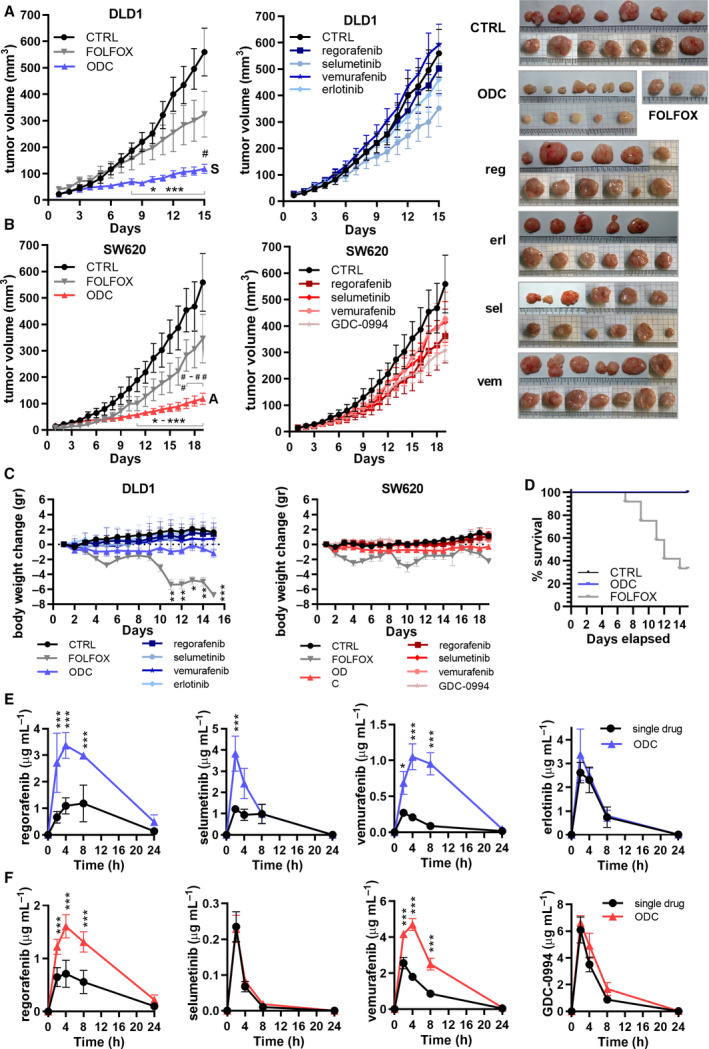
DLD1‐specific ODC efficacy in vivo and pharmacokinetics of the drugs composing. The ODC. a. DLD1 and b. SW620 tumor growth curves and representative images of subcutaneously implanted tumors in Swiss Nu/nu mice after 15 or 19 days of daily treatment, respectively, in N = 2 independent experiments, respectively, with CTRL, ODC, and FOLFOX (left graph) and corresponding monotherapies (right graph). Synergy (S) and additivity (A) of the overall combination is indicated for DLD1 and SW620, respectively. For DLD1, significance was observed for ODC (n = 16) compared to CTRL (sham, n = 13), FOLFOX (n = 4), 15 mg/kg regorafenib (n = 12), 12.5 mg/kg erlotinib (n = 11), 5 mg/kg selumetinib (n = 11), and 75 mg/kg vemurafenib (n = 13). For SW620, significance was observed for ODC (n = 16) compared to CTRL (sham, n = 7), FOLFOX (n = 4), 30 mg/kg regorafenib (n = 8), 0.2 mg/kg selumetinib (n = 6), 75 mg/kg vemurafenib (n = 7), and 10 mg/kg GDC‐0994 (n = 11). FOLFOX was administered as 6 mg/kg oxaliplatin two hours before administration of 90 mg/kg leucovorin and 50 (DLD1) or 25 (SW620) mg/kg 5‐fluorouracil at day 1 and a repeat of 5‐fluorouracil dosing on day 2. c. Weight loss of ODC‐, CTRL‐, and FOLFOX‐treated mice over time of mice with DLD1 and SW620 tumors, respectively. d. Survival of ODC‐, CTRL‐, and FOLFOX‐treated mice with DLD1 subcutaneous tumors over time. The survival of FOLFOX‐treated mice is reduced to n = 4 at the experimental endpoint. e,f. Drug concentrations in blood serum at 2h, 4h, 8h, and 24h post‐treatment with the ODC or the corresponding monotherapies of mice carrying subcutaneous DLD1 or SW620 tumors (N = 4). Error bars represent the SEM (a,b) or SD (c,e,f) and significance of *p < 0.05, **p < 0.01, and ***p < 0.001 represent the comparison with the ODC (a,b), no weight loss (g,h) or the comparison between the drug administered as single drug or as part of the ODC (e,f) using a two‐way ANOVA with post hoc Dunnett’s (a,b,c) or Sidak’s (e,f) multiple comparisons test
