Abstract
Objectives:
To model the contribution of implant material and insertion trauma on loss of acoustic hearing after cochlear implantation in an appropriate animal model.
Methods:
Sixty-five C57B1/6J mice underwent unilateral implantation with implant grade materials: 2 implant grade silicones and a third uncoated platinum wire. A sham surgery group was included as a control. Serial auditory brainstem response (ABR) thresholds and distortion product otoacoustic emissions (DPOAEs) were used to discern effects on hearing over 22 weeks. Histologic measurements of damage to the organ of Corti and spiral ganglion were correlated with degree of hearing loss and material type.
Results:
Organ of Corti damage correlated with rate of hearing loss soon after implantation (0–2 weeks) but not subsequently (2–22 weeks). Organ of Corti damage did not depend on implant type and was present even in sham surgery subjects when hearing was severely damaged. Spiral ganglia appeared unaffected. There was no evidence of an inflammatory or toxic effect of the materials beyond the site of implant insertion.
Conclusions:
Hearing loss and cochlear damage appear to be related to insertion trauma, with minimal effect on delayed hearing loss caused by different materials. In the C57B1/6J mouse model, the sensory epithelium appears to be the location of damage after cochlear implantation.
Keywords: silastic, cochlear implantation, hearing loss high frequency, mice inbred C57BL
Introduction
Cochlear implants (CIs) restore auditory perception to individuals with severe to profound hearing loss by bypassing hair cell transduction and directly stimulating surviving neural elements of the auditory nerve. Hearing preservation CIs have been investigated as a means of maintaining the viability of surviving hair cells in the mid to apical cochlea and allowing for combined acoustic and electric transduction of auditory signals.1 This method allows for significant gains over electric-only stimulation.2
Hearing preservation implants are largely successful at retaining useful acoustic hearing in recipients. Despite this, some ipsilateral loss of acoustic hearing is expected. Most recipients lose some hearing with the operative trauma associated with implantation (10–13 dB average across frequencies). Atraumatic techniques are obligatory.3 There is a subset of patients (20% or more) for whom this hearing loss continues to progress after activation.3 The timeline of hearing loss in some hearing preservation implantees suggests that high amplitude electrical stimulation in combination with high acoustic noise levels may exacerbate ipsilateral hearing loss.3 Alternately, scar formation and fbrosis have been posited as mechanisms for delayed loss of hearing, either due to changes in cytoarchitecture or fluid dynamics.4–6 The extent to which the implant stimulates a systemic immune response or “foreign body” granulomatous response has yet to be elucidated.7,8
In this study, we sought to evaluate the hypothesis that ongoing effects from an indwelling foreign body may cause delayed damage to cochlear cytoarchitecture, resulting in worsening hearing, independent of noise and electrical stimulation. We used a previously validated C57Bl/6J mouse model9 as a mimic for the condition of human presbycusis that hearing preservation implantation most commonly seeks to treat.10,11 Materials used in hearing preservation CIs were varied to dissociate the degree to which implant material and operative trauma play a role in late hearing loss. Histological effects on the scala tympani (ST), organ of corti (OC), and spiral ganglion (SG) were assessed.
Materials and Methods
Animals and Groups
Sixty-five C57Bl/6J mice were used in this study, 54 of which survived the full 22 weeks. The materials included were platinum (platinum wire group) and 2 different grades of silicone: (hereafter) Silastic 1, a 30 Shore A durometer liquid silicone rubber, and Silastic 2, a 50 Shore A durometer high consistency rubber peroxide-cure silicone elastomer, both commercially available materials in current use as hearing preservation implant silicones. The silicone samples were coated onto the same platinum wire as used in the platinum wire group. The devices are depicted in Figure 1. Fourteen animals were implanted with a Silastic 1 implant, 14 animals were implanted with Silastic 2, 18 were implanted with platinum wire, and 19 underwent a sham operation in which a round window cochleostomy was performed with a platinum wire but no implant placed (sham). Based on the ratio of hearing preservation cochlear implant electrode array diameter to the adult human round window (~0.35/1.75 mm), Silastic 1 and Silastic 2 implant dimensions were sized appropriately for the mouse round window (RW) (0.15~0.7 mm).12,13 The platinum wire implants were of a significantly smaller diameter (0.08 mm vs 0.15 mm) and possessed slightly different mechanical properties in that they were stiffer, more moldable, and less rounded at the tip. The implants were placed in the ST via a modified, minimally invasive dorsal approach.9 The contralateral ear was used as a nonoperative control for physiologic testing and histologic analysis. Microscopic otoscopy was used to rule out middle ear effusion or hemotympanum. This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 USC et seq); the Institutional Animal Care and Use Committee of the University of Iowa approved all procedures.
Figure 1.
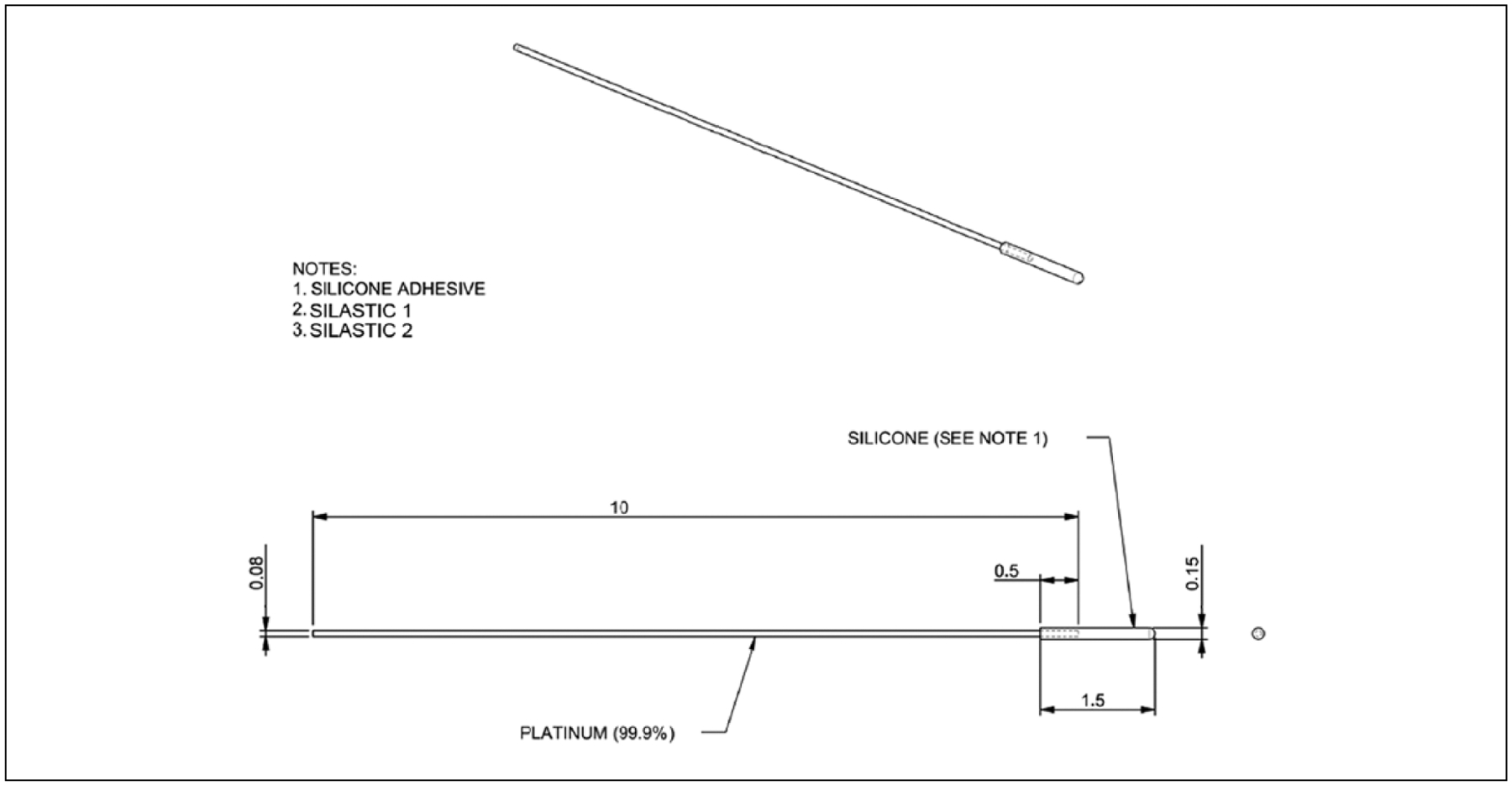
Schematic displays the mock electrode design. All measurements are in millimeters. Silastic 1 and Silastic 2 refer to the 2 silicates used in this study. The uncoated platinum wire was used for the platinum wire group and to open the round window without permanent insertion for sham surgery.
Physiologic Tests
The hearing status of each animal was assessed using click-evoked auditory brainstem response (ABR) and distortion product otoacoustic emissions (DPOAEs) prior to surgery and at 2, 4, 10, 16, and 22 weeks postoperatively. Details are described in Soken et al.9 The ABRs and DPOAEs were measured using an Etymotic Research ER10B+ probe microphone (Elk Grove, Illinois, USA) coupled to 2 Tucker Davis Technologies MF1 Multi-Field Magnetic Speakers (Alachua, Florida, USA). Stimulus presentation and recording were controlled using custom software running on a PC connected to a 24-bit external sound card (Motu UltraLite mk3). The ABRs were amplified, filtered, and recorded using standard signal-averaging techniques. The DPOAEs were measured using frequency glides as described in Soken et al,9 with 2f1-f2 frequencies from 1 to 18 kHz.
Surgical Procedure
Details of the surgical protocol are described in Soken et al.9 Briefly, a tympanotomy is created using a 0.7 mm diamond burr, exposing the RW. The RW membrane is perforated, and the implant is inserted to a depth of about 2 mm. After perforation with a platinum wire, no implant is placed in cochleae of the sham surgery group. To minimize leaking of perilymphatic fluid, fascia is packed into the tympanotomy hole, thus sealing the cochleostomy site.
Histology
Following final determination of DPOAE and ABR responses, temporal bones were preserved by intracardiac perfusion of 0.9% saline followed by 1.5% glutaraldehyde and 2.5% paraformaldehyde in 0.1 M phosphate buffer. Cochleae were dissected from surrounding bone, stapes and implants were carefully removed to facilitate sectioning, and fixative was gently flushed through the scalae. Specimens were then immersed in fresh fixative overnight, post-fixed in 1% osmium tetroxide with 1.5% potassium ferricyanide, decalcified in 0.1M EDTA (pH 7.5), dehydrated in graded alcohols, and embedded in epoxy resin. The cochleae were divided in the mid-modiolar plane with a cut through and perpendicular to the round window. The 1 pm thick perimodiolar sections were mounted on glass slides, stained with azure II and methylene blue, cover-slipped, examined, and photographed with a Nikon Labophot photomicroscope. Micrographs were then analyzed to determine the condition of cochlear structures including the OC, SG, and peripheral axons (PA). Entire sections were photographed at a magnification of 4× to evaluate the SG. A 20× micrograph was then taken of each section at the level of the most basal profile of the OC and associated PA.
Scala Tympani.
Cochlear sections adjacent to the round window membrane were examined qualitatively for evidence of fibrosis or significant granulomatous reaction. They were assessed for presence or absence of a fibrotic capsule.
Organ of Corti.
The OC was evaluated in 20× photomicrographs using a grading scale adapted from Leake et al.14 Five representative subjects were chosen within each subject group. Each section was assigned an ordinal score from 1 to 5. These categories are described and depicted in Figure 2.
Figure 2.
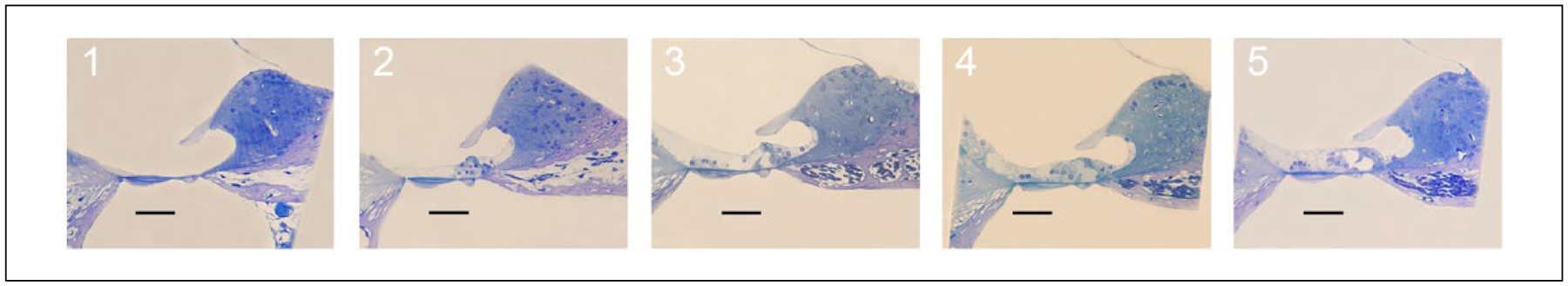
Organ of Corti (OC) ordinal grading system is depicted with representative histologic sections at 20× magnification. Scale bar = 100 μm. 5 = normal OC architecture, presence of inner and outer hair cells, inner hair cell (IHC) cilia visible. 4 = normal OC architecture, presence of IHC and outer hair cell (OHC), no IHC cilia visible. 3 = abnormal IHC and/or OHC, tunnel of Corti open. 2 = tunnel of Corti collapsed. 1 = single cell layer.
Spiral Ganglion and Peripheral Axons.
The density of spiral ganglion neural elements in Rosenthal’s canal (SG) and the osseous spiral lamina (PA) was determined using Image J software. Rosenthal’s canal was outlined manually in 4× micrographs to determine the area to be analyzed. Color threshold of the image was adjusted to include only neural elements, and SG density calculated by the software. The osseous spiral lamina (OSL) was outlined in 20× micrographs and analyzed for PA density in the same way. Only mid-modiolar sections were selected for this analysis (n = 3–5 per group). The method is demonstrated in Figure 3A Rosenthal’s canal (RC) and Figure 3B (OSL).
Figure 3.
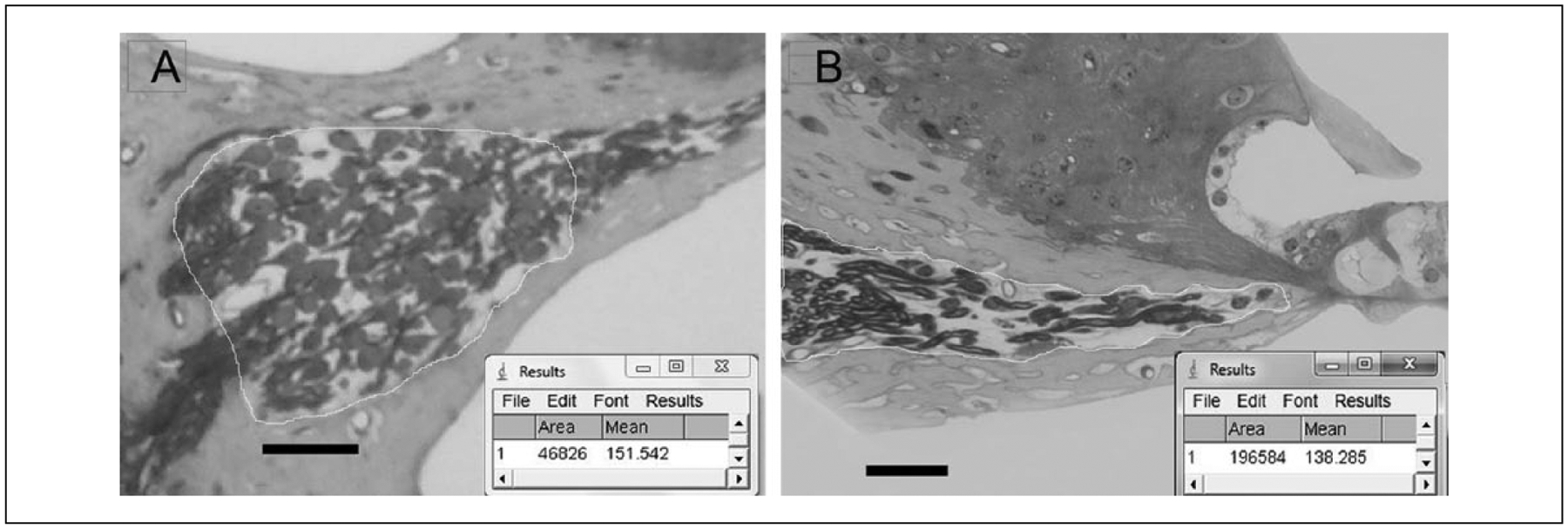
Method to measure neural density. (A) Image J screen capture depicting density measurement for spiral ganglion neuron (SGN) cell bodies in Rosenthal’s canal. Scale bar = 50 μm. (B) Image J screen capture depicting density measurement for peripheral axons in the osseous spiral lamina. Scale bar = 50 μm.
Data and Statistical Analysis
The ABR, DPOAE threshold data, and histology measures were stored and managed using Excel software (Microsoft, Redmond, Washington, USA). Differences in mean ABR thresholds were tested for statistical significance using a 1-way analysis of variance (ANOVA) with post hoc Kruskal-Wallis analysis using SigmaStat software (Ashburn, Virginia, USA). Two group comparisons were conducted using Student’s t test assuming the groups met assumptions for normality. Statistical significance was assigned to comparisons for which P < .05. Error bars, where depicted, represent standard error (SEM).
Results
Animal survival rate did not differ significantly between treatment groups: Silastic 1, 11 of 14 (79%); Silastic 2, 14 of 14 (100%); wire, 15 of 18 (83%); and sham, 14 of 19 (74%). One sham subject was excluded due to development of otitis media. Auditory testing data were accrued throughout the study period. Histology was only collected from animals that survived the full study period.
ABR
Mice implanted with Silastic 1 had, on average, a 49.8 ± 3.8 dB increase in threshold between preoperative measurement and week 22 postoperatively (Figure 4A). Of that loss, 40.3 dB occurred within 2 weeks after implantation. An average of 10.1 dB was lost between week 2 and 22 at a rate of 0.5 dB/wk. Mice implanted with Silastic 2 had an average loss of 58.1 ± 2.2 dB at the end of the study period. Of that loss, 50.8 dB occurred within 2 weeks after implantation. In addition, 7.3 dB were lost between weeks 2 and 22 at a rate of 0.4 dB/wk. Mice implanted with just a platinum wire have an overall 46.0 ± 4.4 dB increase in threshold, with 27.8 dB increase within 2 weeks after implantation and 18.2 dB thereafter (0.9 dB/wk). Mice undergoing a sham operation still lost hearing over the course of the experiment: 28.7 ± 5.4 dB, with 16.2 dB increase in threshold within 2 weeks after opening of the round window and 11.4 dB increase thereafter (0.6 dB/wk). ABR thresholds over the 22 weeks are depicted in Figure 4A. Differences between rates were significant in that mice with less hearing loss during the initial postoperative period lost more subsequently—evidencing a ceiling effect.
Figure 4.
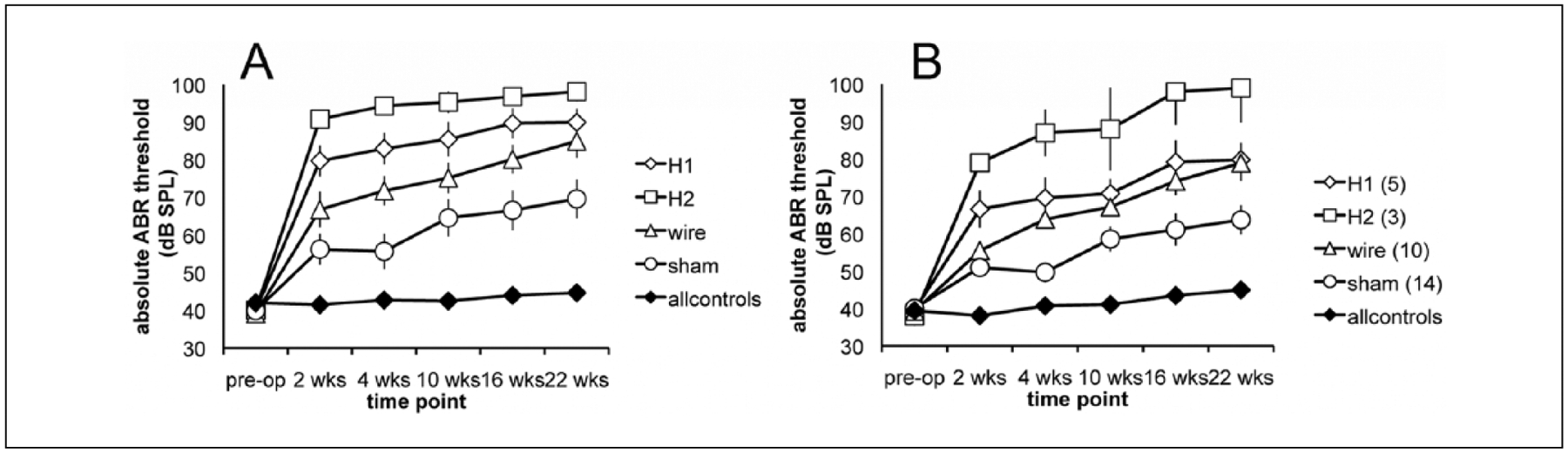
Threshold increase is shown for all experimental conditions including Silastic 1, Silastic 2, wire, sham, and controls. (A) All subjects included irrespective of distortion product otoacoustic emission (DPOAE) retention after insertion. Threshold increased more for larger implants in the first 2 weeks after insertion (*0–2 weeks, ANOVA, P < .0001). Slope of increase from 2 to 22 weeks did not differ significantly between groups. Error bars reflect standard error (SEM). (B) The DPOAE-positive subjects were analyzed. Differences in threshold were also significant at 2 weeks (ANOVA, P < .0001), but slope of threshold increase was likewise similar thereafter.
DPOAE
One hundred percent of animals had reliable DPOAEs above the noise floor (>6 dB signal-to-noise ratio) at baseline. At 2 weeks postoperatively, percentage of detectible DPOAE were as follows: Silastic 1, 31%; Silastic 2, 16%; wire, 58%; sham, 75%. Middle ear effusion was detected in 1 animal but was not found in the remainder either on microscopic otoscopy or histology. Those animals that lost DPOAEs after implantation had comparable increases in ABR threshold at the 2-week check irrespective of implant type—Silastic 1, 49.7 dB; Silastic 2, 53.5 dB; wire, 51.0 dB; sham, 51.0 dB—and were in each instance significantly worse than animals in the same cohorts that retained DPOAEs (Figure 5).
Figure 5.
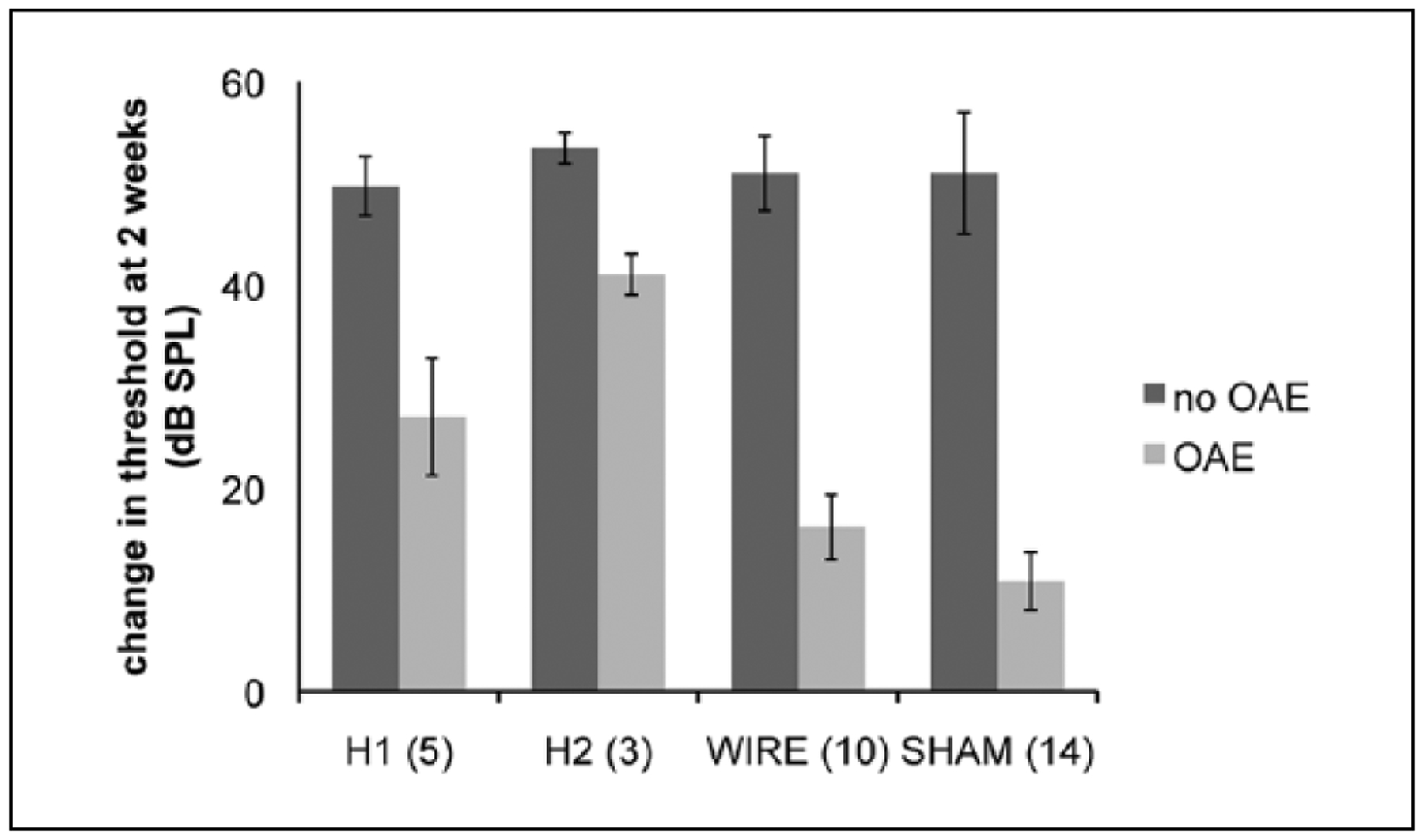
Auditory brainstem response (ABR) threshold increase over the first 2 weeks post-implantation was compared between animals that retained distortion product otoacoustic emissions (DPOAEs) and those that did not in each group. Threshold increase was essentially equivalent in those animals for which DPOAE responses fell below the noise floor (~50 dB). Even sham surgery subjects that lost DPOAE responses suffered this high degree of hearing loss. Conversely, animals that retained OAEs had threshold increases more commensurate with the size of the implant and degree of trauma (Silastic 1, 40 dB; Silastic 2, 61 dB; wire, 40 dB; sham, 23 dB).
For comparison, we analyzed acoustic ABR results from the subset of animals that retained DPOAEs following implantation as depicted in Figure 4B. Results were similar, except for a higher degree of variation, presumably due to lower numbers. Animals implanted with Silastic 1 lost an average of 40.2 ± 5.6 dB in the first 2 weeks after implantation and 0.7 dB/wk subsequently (n = 5). Silastic 2 subjects lost 61 ± 6.1 dB total on average. These subjects also suffered worse hearing loss within 2 weeks after implantation that slowed to 1 dB/wk average rate of loss after week 2. Animals implanted with a platinum wire alone lost 39.6 ± 4.8 dB in the first 2 weeks after implantation and 1.2 dB/wk from 2 to 22 weeks. Total hearing loss (HL) in ears that underwent sham surgery was 23.0 ± 4.4 dB over the total test period with a 10.8 dB loss in the first 2 weeks and a rate of 0.6 dB/wk thereafter.
The rate of HL related to the degree of operative trauma from a larger implant (Figure 6A). Silicon implants (diameter 0.15 mm) Silastic 1and Silastic 2 precipitated a higher rate of HL (mean = 20.1 and 25.4 dB/wk, respectively) than both platinum wire (diameter 0.08 mm) and sham surgery conditions (mean = 13.9 and 8.1 dB/wk, respectively, ANOVA P < .0001). At later intervals, differences in rate of HL are physiologically negligible and statistically significant only from week 4 to 10 wherein higher rate was seen in the sham surgery group (mean = 1.3 dB/wk, ANOVA P = .023), again displaying the ceiling effect. The same analysis, when performed for only those animals that retained DPOAEs after implantation, yielded similar results within the first 2 weeks after surgery (ANOVA P < .001) and higher rate of HL for Silastic 2 and wire conditions in the 2- to 4-week interval (Silastic 1, 1.5; Silastic 2, 4; wire, 4.2; sham, −0.6 dB/wk, P < .0003, Figure 6B).
Figure 6.
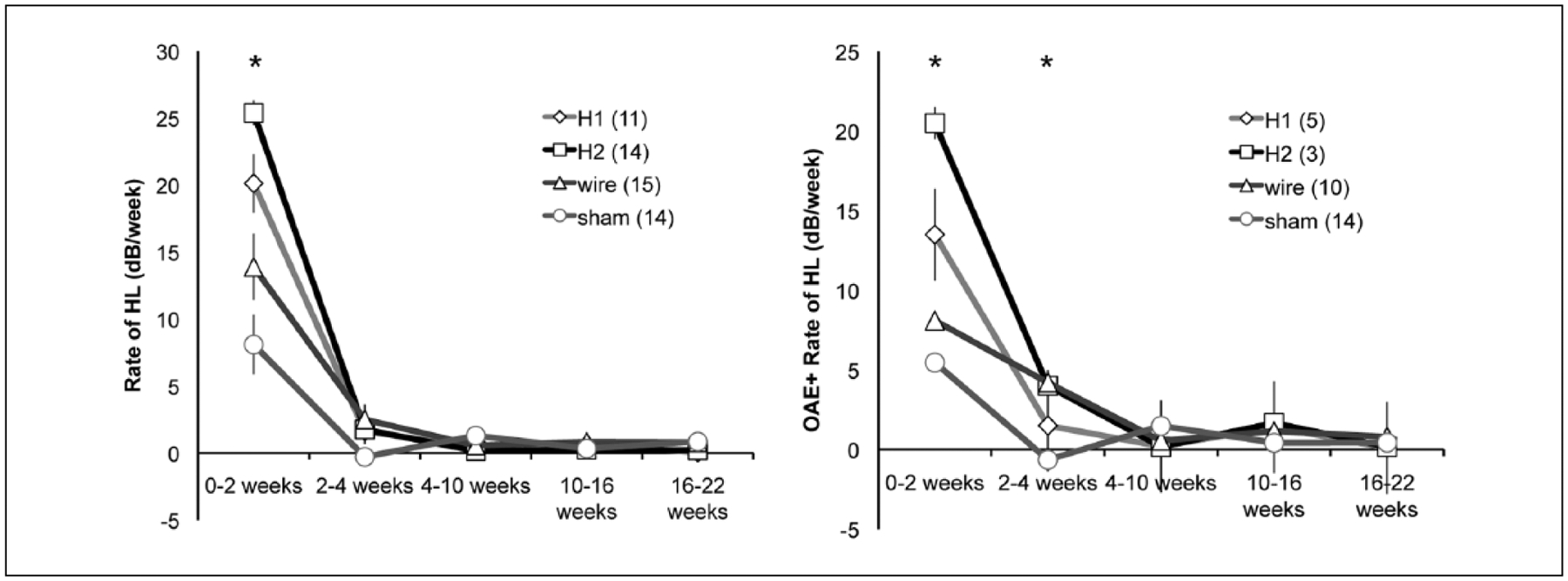
Rate of hearing loss (dB/wk) is depicted across serial intervals for (A) all subjects and (B) only those subjects that retained distortion product otoacoustic emissions (DPOAEs) after implant insertion. Numbers per subject group are shown in the figure legends. Rate of hearing loss differed between groups after insertion (0–2 weeks) but was nearly identical subsequently. When only DPOAE-positive animals were included, Silastic 2 and wire conditions had small but significant differences in rate of hearing loss at 2 to 4 weeks from the Silastic 1 and sham conditions (Silastic 1, 1.5; Silastic 2, 4; wire, 4.2; sham, −0.6 dB/wk, P < .003).
The rate of hearing loss was examined as a function of degree of OC damage at sacrifice (scale 1 to 5) both from baseline to the 2 weeks post implantation, putatively associated with initial implantation trauma, and from 2 to 22 weeks, which we considered delayed. The degree of OC damage was correlated with rate of hearing loss after implantation with grade 1 (flat epithelium) cochleae associated with loss of 17.25 dB/wk in the first 2 weeks; grade 2, 14.25 dB/wk; grade 3, 15.6 dB/wk; grade 4, 2.6 dB/wk; grade 5 (normal organ of Corti), 4.75 dB/wk. Thus, higher rate of hearing loss in the first 2 weeks was correlated with greater degree of OC damage (ANOVA, P = .023). Rates of hearing loss subsequently (2–22 weeks) were no different between different OC groups (Figure 7).
Figure 7.
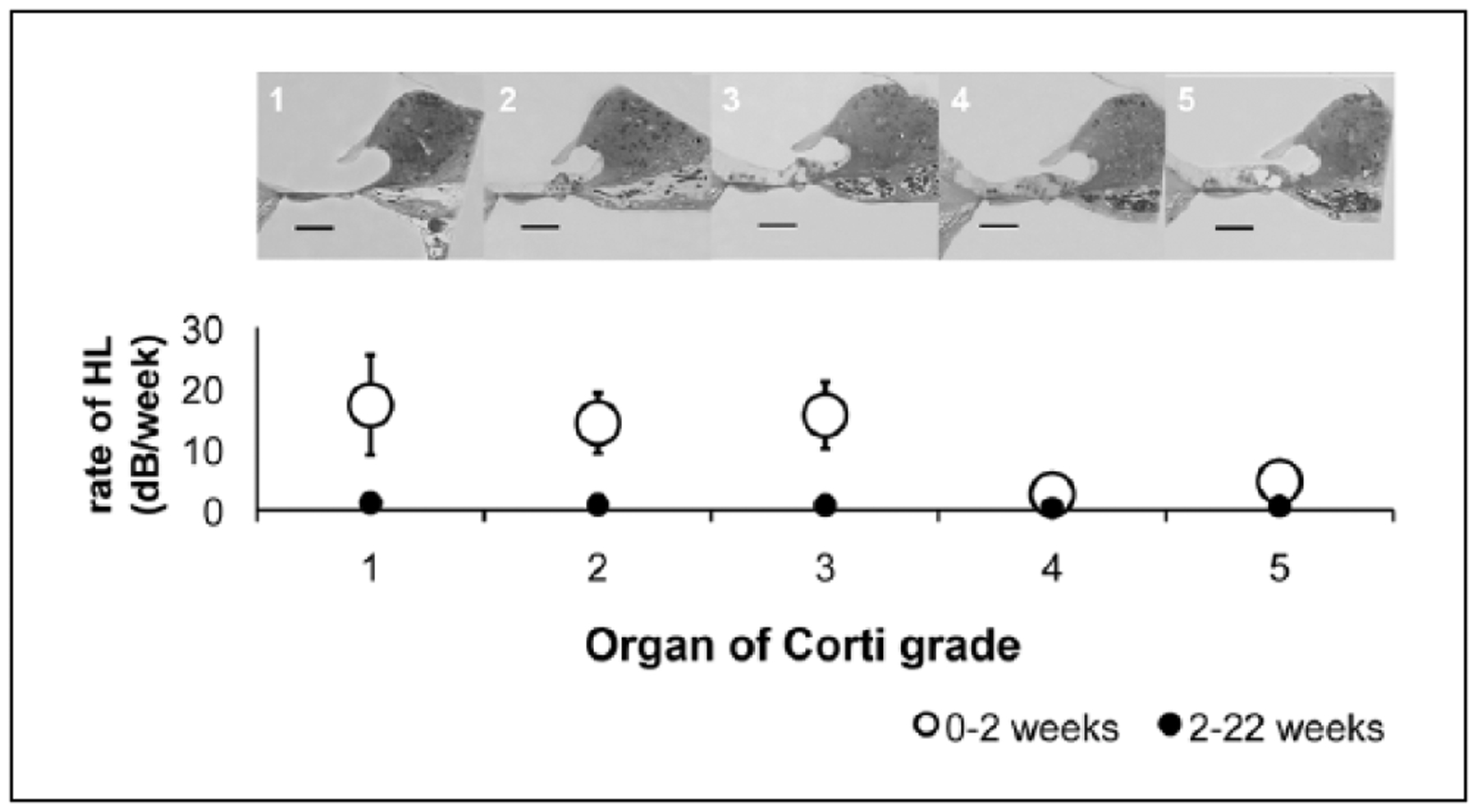
The rate of hearing loss was examined as a function of degree of organ of Corti (OC) damage at sacrifice (scale 1 to 5) both from baseline to the 2 weeks post-implantation (open circles) and from 2 to 22 weeks (closed circles). Higher rate of hearing loss in the first 2 weeks was correlated with greater degree of OC damage (ANOVA, P = .023). Rates of hearing loss subsequently (2–22 weeks) were no different between different OC groups. Scale bar = 100 μm.
In contrast, OC grade was not correlated with material. No significant differences were found in ultimate histology between the implant subgroups including sham and wire conditions (Figure 8, ANOVA, P = .8). Control ears had a tendency to have a higher grade than contralateral operated ears, but these comparisons did not reach significance (Student’s t test, P > .05).
Figure 8.
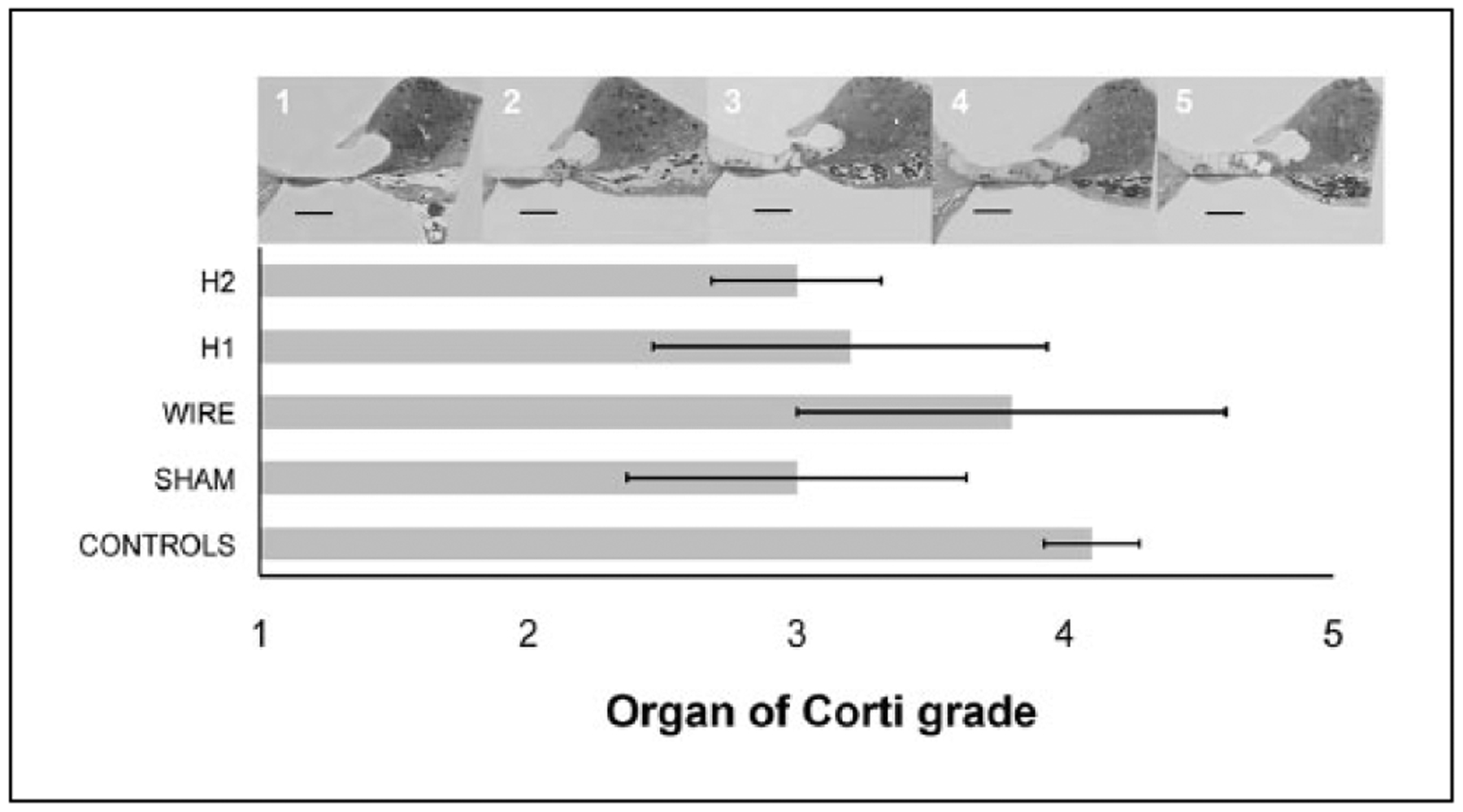
Organ of Corti grade (1–5) was not highly correlated with implant type. No significant differences were found in ultimate histology between the implant subgroups including sham and wire conditions (ANOVA, P = .8). Control ears had a tendency to have a higher grade than contralateral operated ears, but these comparisons did not reach significance (Student’s t test, P > .05). Scale bar = 100 μm.
Comparison of spiral ganglion cell body density and PA density between test and contralateral ears as well as between conditions was undertaken using the technique described previously. Five subjects were compared from each subgroup. There were no significant differences in neural density in Rosenthal’s canal or the OSL among subgroups (Figures 9A, 9B). Evaluation of fibrosis was chellenging due to the potential disturbance of thin fibrotic tissue capsules upon removal of the implants. Fibrosis was observed in cochleae implanted with silicone but not those implanted with platinum wires or control, sham cochleae. There was no apparent difference in the intracochlear fibrosis between silicone types, and there was no fibrosis or evidence of inflammation apical to the area of the Important. Representative sections are displayed in Figure 10.
Figure 9.
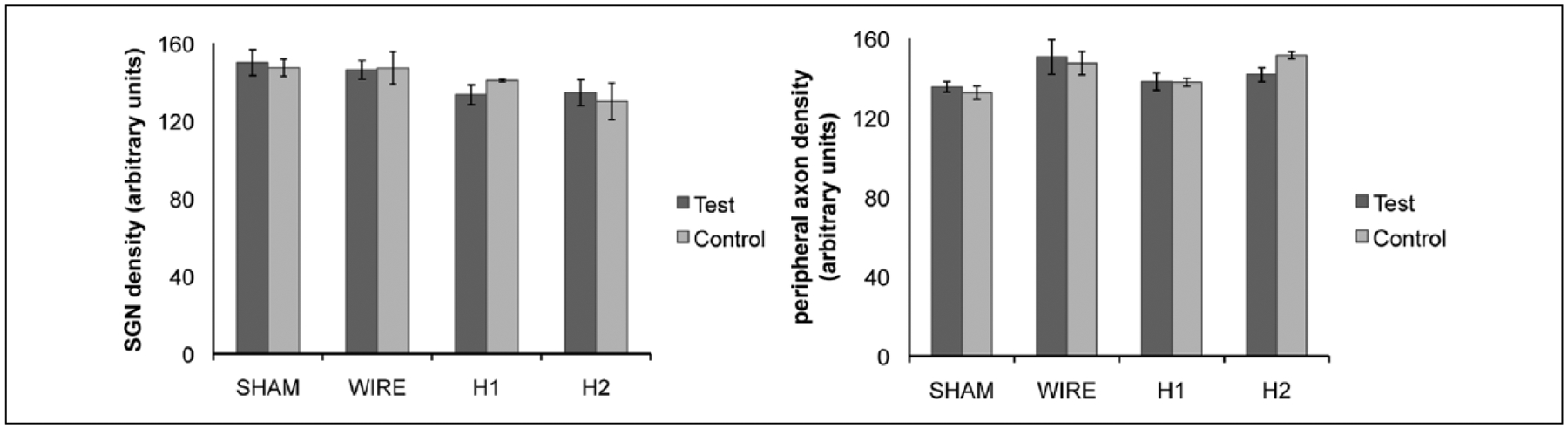
(A) Spiral ganglion and (B) peripheral axon density were compared across all conditions. There were no significant differences in cell body or peripheral process density among subgroups.
Figure 10.
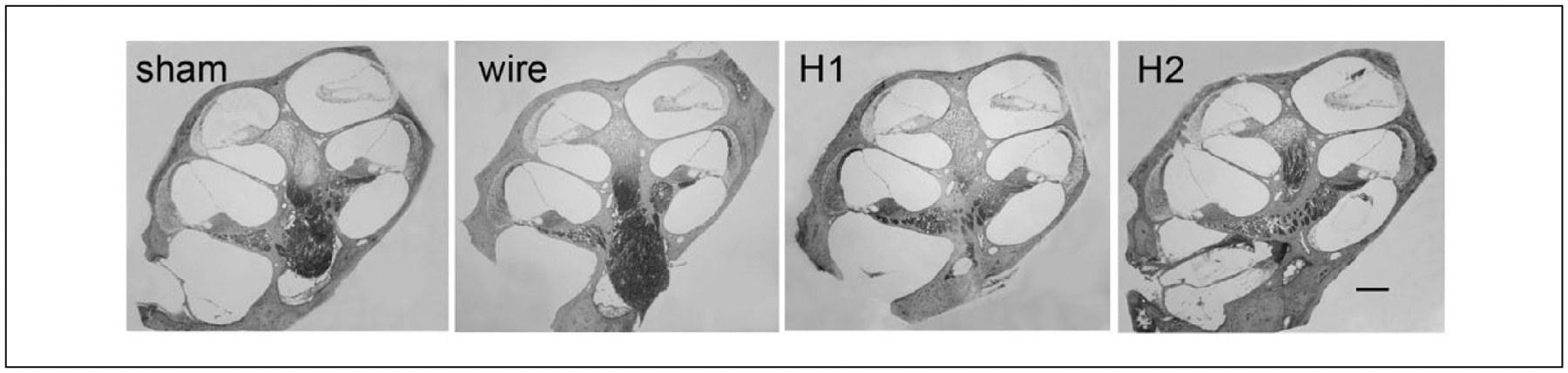
Mid modiolar sections were examined qualitatively for evidence of scala tympani fibrosis. Examples are depicted (scale bar = 200 μm). Only 3 subjects with Silastic implants had a significant fibrous capsule (2 Silastic 2, 1 Silastic 1) evident in the scala tympani, with no scarring apical to the Silastic implant. No sham or wire subjects developed scarring.
Discussion
Profound loss of acoustic hearing after hearing preservation cochlear implantation may limit the usefulness of the device in certain situations. This post-implantation hearing loss is likely multifactorial, complicating our ability to determine its etiology clinically. Implantation trauma is important and may be predictive.15 The degree of posttraumatic inflammation in the cochlea is likely to contribute to hearing loss susceptibility.5–7 Additional factors may be related to excite-toxicity of high intensity acoustic and electric stimuli.3 Here we analyzed the effect of cochlear implantation itself, independent of acoustic and electric stimuli.
We used C57B1/6J mice as this strain suffers accelerated high-frequency sensorineural hearing loss starting with loss of DPOAEs similar to patients who are candidates for hearing preservation electrode arrays.16 It is likely that these animals are more susceptible to post-implantation hearing loss compared to other strains of mice. The mice were implanted with hearing preservation CI materials, and changes in acoustic thresholds and hair cell responses were determined over time and correlated with histological changes. Analysis reveals that after accounting for surgical trauma by eliminating subjects who lost DPOAEs, cochlear damage correlates with degree of hearing loss due to implantation irrespective of implant material. Furthermore, the rate of hearing loss after implantation did not appear to vary between implant types after the initial post-implantation HL determined at 2 weeks after implantation. These data suggest that there is a limited response in the cochlea to implant materials following implantation trauma.
Implantation trauma was sufficient to cause considerable loss of hearing, including loss of DPOAEs in a number of subject animals. This was further supported by the high correlation between OC damage with loss of hearing after implantation. This high degree of loss within 2 weeks following implantation made subsequent analysis of long-term impact of indwelling materials on acoustic hearing subject to ceiling effects. Our data on animals in which DPOAEs were preserved reveal no significant differences in delayed hearing loss between subject groups, although variability was high. These data support efforts to mitigate insertion trauma as a critical step in preserving hearing after implantation.15,17,18
Our data suggest that implant material itself does not significantly contribute to progressive hearing loss in hearing preservation efforts. However, our ability to reject the null hypothesis is limited by sample size when we exclude animals that lost DPOAEs with insertion.
Implantation trauma has been studied extensively. The cochlear microphonic and endoscopy have been trialed in both Mongolian gerbils and humans in order to measure and minimize the degree of damage as it occurs.19,20 Intratympanic and systemic steroids have been suggested to dampen the secondary damage caused by the post-insertion inflammatory cascade.21–28 While the mouse model, particularly the C57Bl/6J, may be particularly susceptible to loss of hearing with implantation secondary to its anatomic constraints and susceptibility of its sensory epithelium to reactive oxygen species,16,29,30 our data validate the importance of efforts to mitigate post-insertion inflammation.
Neither the fibrotic tissue in the scala tympani nor the density of neural elements of the OSL or RC appeared to be correlated to a high degree with loss of hearing. These data do not support the hypothesis that scar tissue and change in fluid dynamics from the implant are the major factors in late loss of hearing.4 This is in contrast to findings in guinea pigs and recent work in mice.6,31 Furthermore, our data point to the sensory epithelium as an area particularly sensitive to damage from inflammation after implantation. Acoustic and electrical inputs, not examined here, may cause additional harm within this inflammatory milieu, either by oxidative damage to the sensory epithelium or delayed synaptic damage.3,32,33 Further work is needed to understand the mechanisms that lead to late ipsilateral hearing loss after implantation.
Limitations
Animals with loss of DPOAEs demonstrated maximum threshold shift (~50 dB) independent of experimental condition. This loss represents a major limitation to the study in that it is difficult to dissociate middle from inner ear effects. Histology appears to bear out the premise that the most severe loss of hearing (including loss of DPOAEs) occurred in those with the worst cochlear damage. Attempts to study loss of hearing are predicated on having additional hearing to lose. We posit that retention of DPOAEs is a good inclusion criterion for future studies in the mouse model.
Conclusions
Animal models of residual hearing cochlear implantees are imperfect, often requiring exposure to exogenous ototoxins to mimic the human condition.34 Mouse models are exceptional in that many lose hearing with age in a manner similar to that of presbycusic hearing loss.10,30 In the C57Bl/6J mouse model, rate of hearing loss was equivalent across implant types after implantation. Organ of Corti damage was most closely associated with loss of hearing within 2 weeks after implantation. Retention of OAEs is an important candidacy criterion to measure effect of other variables on late loss of ipsilateral hearing after hearing preservation cochlear implantation.
Acknowledgments
This project would not have been possible without the support and guidance of Dr Bruce J. Gantz and Dr Paul J. Abbas, to whom we would like to extend our gratitude. We’d also like to acknowledge Daniel Smyth for critical review.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by the NIH P30 DC010362 (University of Iowa), J.C.K. was also supported by T32 DC000040 (University of Iowa). Nonfunctional cochlear implant materials used in these experiments were designed in collaboration with, fabricated, and supplied by Cochlear Ltd, Centennial, Colorado, USA.
Footnotes
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Implant materials were provided by Cochlear Ltd. Co-authors Kristien J. Verhoeven and Jonathon R. Kirk are employed by Cochlear Ltd.
References
- 1.Gantz BJ, Hansen MR, Turner CW, Oleson JJ, Reiss LA, Parkinson AJ. Hybrid 10 clinical trial: preliminary results. Audiol Neurootol. 2009;14(suppl 1):32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodson EA, Reiss LA, Turner CW, Gfeller K, Gantz BJ. The hybrid cochlear implant: a review. Adv Otorhinolaryngol. 2010;67:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kopelovich JC, Reiss LAJ, Etler CP, et al. Hearing loss after activation of hearing preservation cochlear implants might be related to afferent cochlear innervation injury [published online April 7, 2015]. Otol Neurotol. PMID: 25955750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi CH, Oghalai JS. Predicting the effect of post-implant cochlear fibrosis on residual hearing. Hear Res. 2005;205(1–2):193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia H, Wang J, François F, Uziel A, Puel JL, Venail F. Molecular and cellular mechanisms of loss of residual hearing after cochlear implantation. Ann Otol Rhinol Laryngol. 2013;122(1):33–39. [DOI] [PubMed] [Google Scholar]
- 6.O’Leary SJ, Monksfield P, Kel G, et al. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hear Res. 2013;298:27–35. [DOI] [PubMed] [Google Scholar]
- 7.Souter M, Eastwood H, Marovic P, et al. Systemic immunity influences hearing preservation in cochlear implantation. Otol Neurotol. 2012;33(4):532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark GM, Clark JC, Furness JB. The evolving science of cochlear implants. JAMA. 2013;310(12):1225–1226. [DOI] [PubMed] [Google Scholar]
- 9.Soken H, Robinson BK, Goodman SS, Abbas PJ, Hansen MR, Kopelovich JC. Mouse cochleostomy: a minimally invasive dorsal approach for modeling cochlear implantation. Laryngoscope. 2013;123(12):E109–E115.23674233 [Google Scholar]
- 10.Ison JR, Allen PD. Low-frequency tone pips elicit exaggerated startle reflexes in C57Bl/6J mice with hearing loss. J Assoc Res Otolaryngol. 2003;4(4):495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ison JR, Allen PD, O’Neill WE. Age-related hearing loss in C57Bl/6J mice has both frequency-specific and non-frequency-specific components that produce a hyperacusis-like exaggeration of the acoustic startle reflex. J Assoc Res Otolaryngol. 2007;8(4):539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santi PA, Rapson I, Voie AH. Development of the mouse cochlea database (MCD). Hear Res. 2008;243:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atturo F, Barbara M, Rask-Andersen H. Is the human round window really round? An anatomic study with surgical implications. Otol Neurotol. 2014;35(8):1354–1360. [DOI] [PubMed] [Google Scholar]
- 14.Leake PA, Hradek GT, Rebscher SJ, Snyder RL. Chronic intracochlear electrical stimulation induces selective survival of spiral ganglion neurons in neonatally deafened cats. Hear Res. 1991;54(2):251–271. [DOI] [PubMed] [Google Scholar]
- 15.Adunka OF, Pillsbury HC, Buchman CA. Minimizing intracochlear trauma during cochlear implantation. Adv Otorhinolaryngol. 2010;67:96–107. [DOI] [PubMed] [Google Scholar]
- 16.Parham K Distortion product otoacoustic emissions in the C57Bl/6J mouse model of age-related hearing loss. Hear Res. 1997;112(1–2):216–234. [DOI] [PubMed] [Google Scholar]
- 17.Roland PS, Wright CG. Surgical aspects of cochlear implantation: mechanisms of insertional trauma. Adv Otorhinolaryngol. 2006;64:11–30. [DOI] [PubMed] [Google Scholar]
- 18.Burghard A, Lenarz T, Kral A, Paasche G. Insertion site and sealing technique affect residual hearing and tissue formation after cochlear implantation. Hear Res. 2014;312:21–27. [DOI] [PubMed] [Google Scholar]
- 19.Choudhury B, Adunka OF, Demason CE, Ahmad FI, Buchman CA, Fitzpatrick DC. Detection of intracochlear damage with cochlear implantation in a gerbil model of hearing loss. Otol Neurotol. 2011;32(8):1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeMason C, Choudhury B, Ahmad F, et al. Electrophysiological properties of cochlear implantation in the gerbil using a flexible array. Ear Hear. 2012;33(4):534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eshraghi AA, Adil E, He J, Graves R, Balkany TJ, Van De Water TR. Local dexamethasone therapy conserves hearing in an animal model of electrode insertion trauma-induced hearing loss. Otol Neurotol. 2007;28(6):842–849. [DOI] [PubMed] [Google Scholar]
- 22.Vivero RJ, Joseph DE, Angeli S, et al. Dexamethasone base conserves hearing from electrode trauma-induced hearing loss. Laryngoscope. 2008;118(11):2028–2035. [DOI] [PubMed] [Google Scholar]
- 23.James DP, Eastwood H, Richardson RT, O’Leary SJ. Effects of round window dexamethasone on residual hearing in a guinea pig model of cochlear implantation. Audiol Neurootol. 2008;13(2):86–96. [DOI] [PubMed] [Google Scholar]
- 24.Eastwood H, Chang A, Kel G, Sly D, Richardson R, O’Leary SJ. Round window delivery of dexamethasone ameliorates local and remote hearing loss produced by cochlear implantation into the second turn of the guinea pig cochlea. Hear Res. 2010;265(1–2):25–29. [DOI] [PubMed] [Google Scholar]
- 25.Connolly TM, Eastwood H, Kel G, Lisnichuk H, Richardson R, O’Leary S. Pre-operative intravenous dexamethasone prevents auditory threshold shift in a guinea pig model of cochlear implantation. Audiol Neurootol. 2011;16(3):137–144. [DOI] [PubMed] [Google Scholar]
- 26.Quesnel S, Nguyen Y, Elmaleh M, et al. Effects of systemic administration of methylprednisolone on residual hearing in an animal model of cochlear implantation. Acta Otolaryngol. 2011;131(6):579–584. [DOI] [PubMed] [Google Scholar]
- 27.Paasche G, Tasche C, Stöver T, Lesinski-Schiedat A, Lenarz T. The long-term effects of modified electrode surfaces and intracochlear corticosteroids on postoperative impedances in cochlear implant patients. Otol Neurotol. 2009;30(5):592–598. [DOI] [PubMed] [Google Scholar]
- 28.Rajan GP, Kuthubutheen J, Hedne N, Krishnaswamy J. The role of preoperative intratympanic glucocorticoids for hearing preservation in cochlear implantation: a prospective clinical study. Laryngoscope. 2012;122(1):190–195. [DOI] [PubMed] [Google Scholar]
- 29.Park SN, Back SA, Park KH, et al. Comparison of cochlear morphology and apoptosis in mouse models of presbycusis. Clin Exp Otorhinolaryngol. 2010;3(3):126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SN, Back SA, Choung YH, et al. α-Synuclein deficiency and efferent nerve degeneration in the mouse cochlea: a possible cause of early-onset presbycusis. Neurosci Res. 2011;71(3):303–310. [DOI] [PubMed] [Google Scholar]
- 31.Mistry N, Nolan LS, Saeed SR, Forge A, Taylor RR. Cochlear implantation in the mouse via the round window: effects of array insertion. Hear Res. 2014;312:81–90. [DOI] [PubMed] [Google Scholar]
- 32.Cheng AG, Cunningham LL, Rubel EW. Mechanisms of hair cell death and protection. Curr Opin Otolaryngol Head Neck Surg. 2005;13(6):343–348. [DOI] [PubMed] [Google Scholar]
- 33.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29(45):14077–14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suberman TA, Campbell AP, Adunka OF, Buchman CA, Roche JP, Fitzpatrick DC. A gerbil model of sloping sensorineural hearing loss. Otol Neurotol. 2011;32(4):544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]


