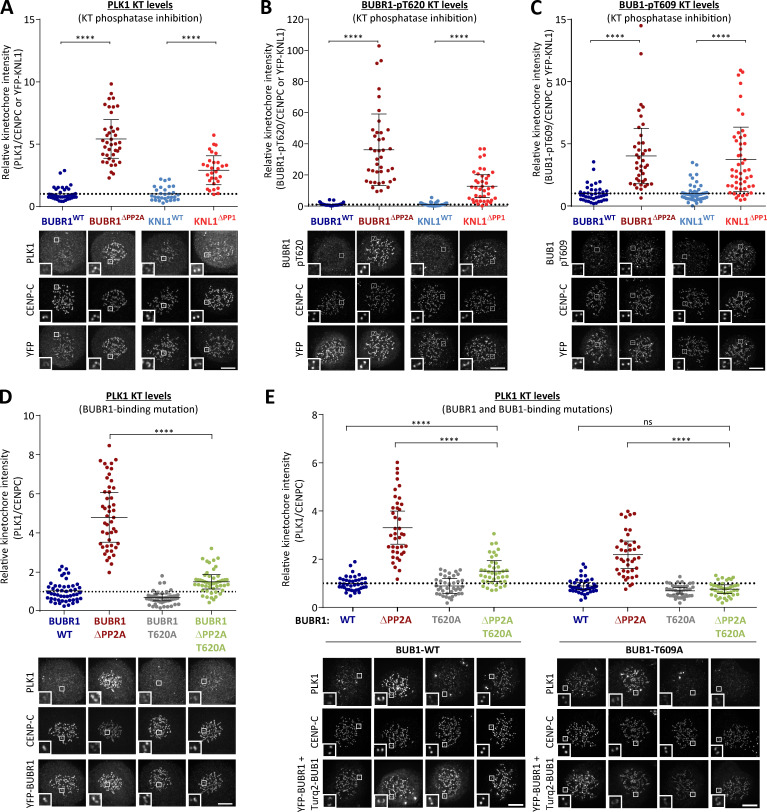
Figure 1.
Kinetochore phosphatases PP1 and PP2A-B56 antagonize PLK1 recruitment to the BUB complex. (A–C) Effect of phosphatase-binding mutants on levels of PLK1 (A), BUBR1-pT620 (B), and BUB1-pT609 (C) at unattached kinetochores in nocodazole-arrested cells. Mean kinetochore intensities from 30–50 cells, three to five experiments. (D and E) Effect of mutating the PLK1 binding site on BUBR1 (pT620) alone (D) or BUBR1 (pT620) and BUB1 (pT609; E) on PLK1 kinetochore levels in nocodazole-arrested BUBR1WT/ΔPP2A cells. Mean kinetochore intensities from 40 cells per condition, four experiments. All values in E are normalized to BUBR1-WT+BUB1-WT control. For all kinetochore intensity graphs, each dot represents a cell, and the error bars display the variation between the experimental repeats (displayed as ±SD of the experimental means). Two-tailed, nonparametric Mann-Whitney unpaired t tests were performed to compare the mean values between experimental groups. Example immunofluorescence images were chosen that most closely resemble the mean values in the quantifications. The insets show magnifications of the outlined regions. Scale bars, 5 µm. Inset size, 1.5 µm. ****, P < 0.0001; ns, nonsignificant.