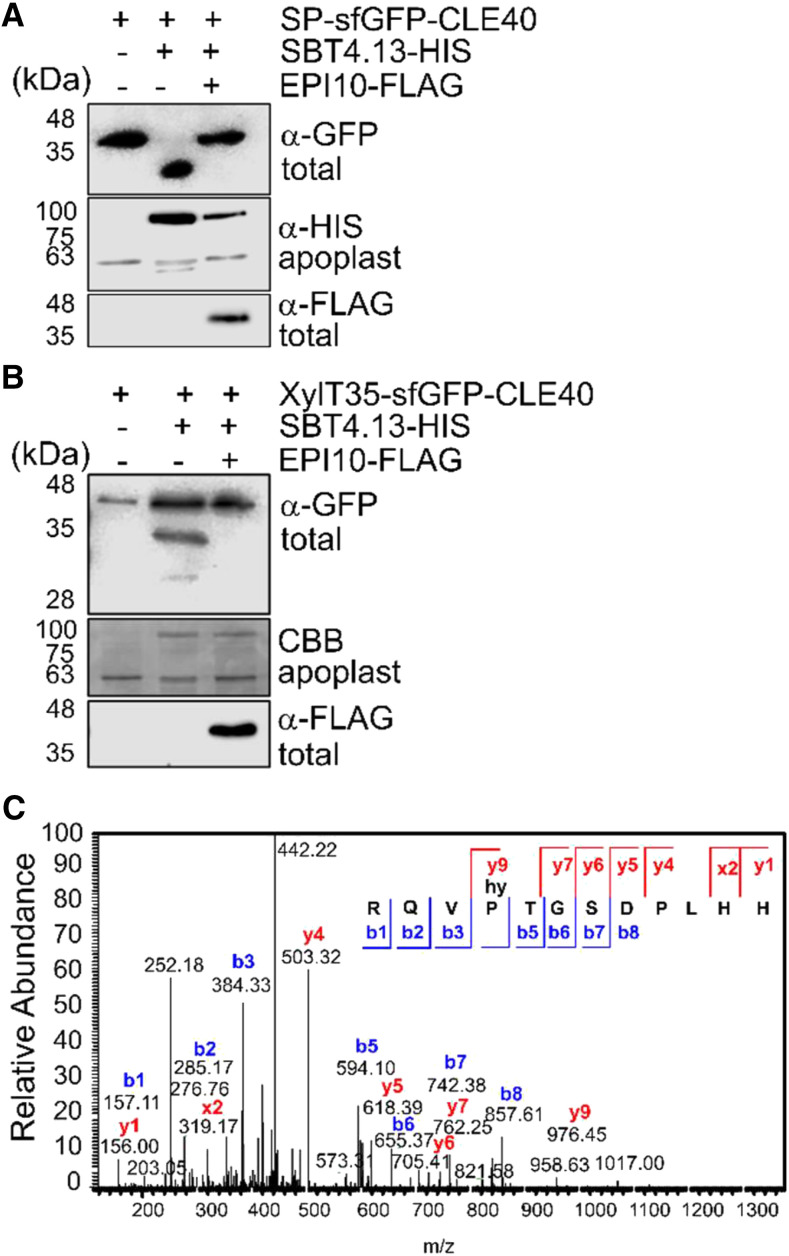
Figure 2.
The CLE40 precursor is processed by SBTs to release matCLE40. A and B, proCLE40 is cleaved by SBT4.13 in the Golgi. A, The CLE40 precursor equipped with an N-terminal signal peptide (SP) and sfGFP tag was transiently expressed in N. benthamiana together with hexa-His-tagged (HIS) SBT4.13 or the empty vector control (SBT1.4 and SBT1.7 in Supplemental Fig. S2). SBT4.13-mediated cleavage of sfGFP-proCLE40 in total leaf extracts is inhibited by coexpression of FLAG-tagged EPI10 targeted to the endoplasmic reticulum. B, EPI-sensitive processing also occurs when sfGFP-CLE40 is retained in the Golgi by the 35-amino acid membrane anchor of Golgi-resident XylT (XylT35). sfGFP-CLE40 and its cleavage products were detected with α-GFP and EPI10-FLAG with α-FLAG antibodies on western blots of total leaf extracts. Expression of SBT4.13 was confirmed by α-His immunoblotting (A) or Coomassie Brilliant Blue (CBB) staining (B) of cell wall extracts. Proteins were separated by SDS-PAGE using the Laemmli buffer system. Notice that the apparent masses of SP-sfGFP-CLE40 and XylT35-sfGFP-CLE40 fusion proteins (approximately 35 and 40 kD, respectively) are higher than the calculated masses (33.2 and 37.4 kD, respectively). MS analysis confirmed the identity of SP-sfGFP-CLE40 and identified the processing site in the cleavage product at, or in close proximity of, the PTG motif. The apparent mass of the SP-sfGFP-CLE40 cleavage product is lower than the calculated mass of 31.6 kD. The large difference in apparent masses of the full-length and processed forms may be caused by posttranslational modifications of the precursor that are missing from the cleavage product. The difference in expected (65 kD) and apparent (80 kD) mass of SBT4.13 is due to glycosylation. C, proCLE40 is processed in planta to release matCLE40 for secretion into the apoplast. The peptide fraction of cell wall extracts from N. benthamiana leaves expressing CLE40-sfGFP was isolated by ultrafiltration (less than 5-kD MMCO) and solid-phase extraction and analyzed by liquid chromatography-electrospray ionization-MS/MS. The fragmentation spectrum is shown for the Mr = 453.896 precursor ion corresponding to P4-hydroxylated matCLE40. The identity of the peptide is confirmed by the ions that were identified in the y (blue) and b (red) series. m/z, Mass-to-charge ratio.