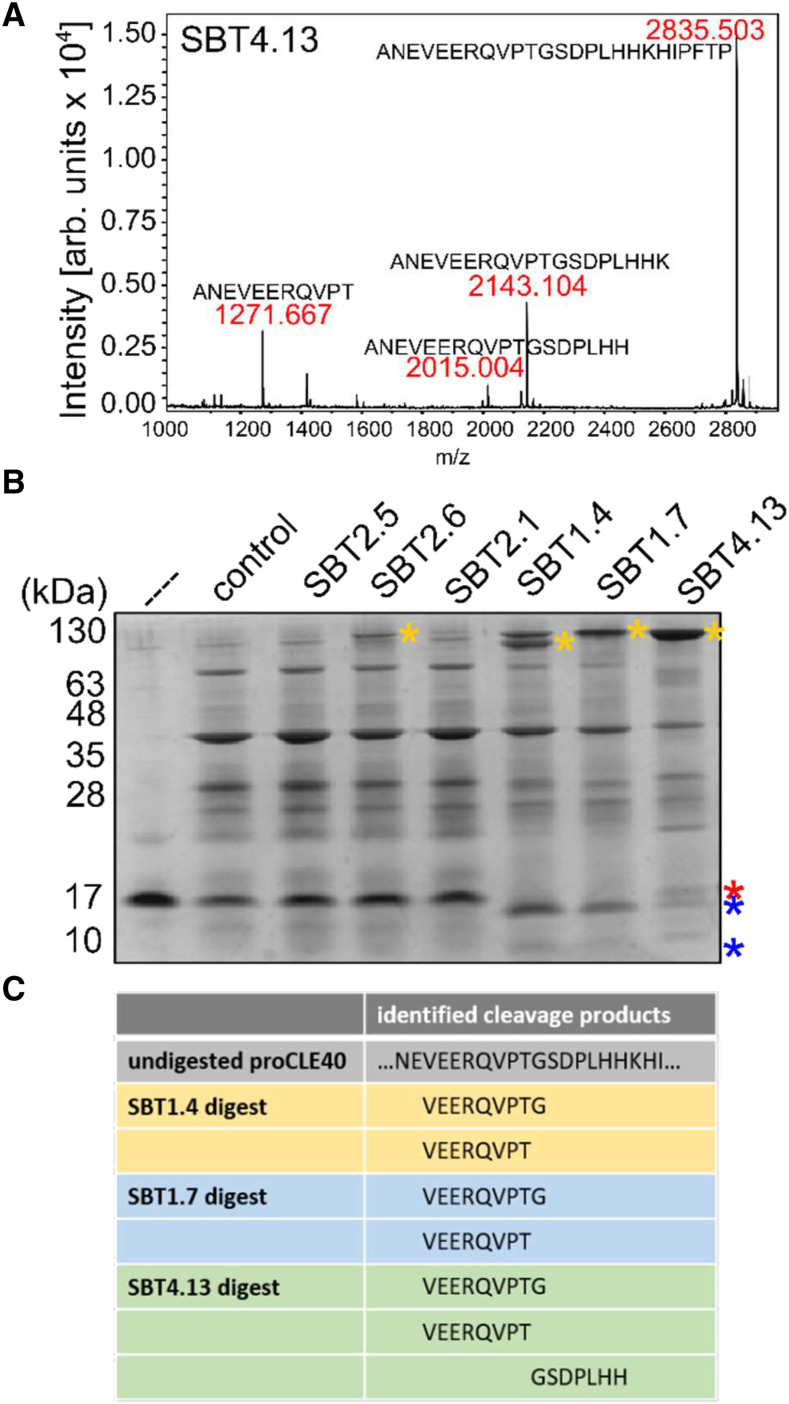
Figure 3.
SBTs cleave the CLE40 precursor in vitro. A, Cleavage of the extended CLE40 precursor peptide by SBT4.13. eCLE40 was digested with affinity-purified SBT4.13 (control reaction in Supplemental Fig. S3A). Reaction products were analyzed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). The mass spectrum shows ion intensities (arbitrary units) and masses for the substrate peptide (m/z = 2,835.503) and three cleavage products. B, Recombinant proCLE40 is processed by SBT1.4, SBT1.7, and SBT4.13. Recombinant proCLE40 (first lane marked with the dashed line) was incubated with cell wall extracts from mock-infiltrated plants (control) and from plants expressing the indicated SBTs. The protein fraction (greater than 10 kD) was analyzed by Tris-Tricine-PAGE. Notice that on Tris-Tricine gels the apparent mass of SBTs (100–130 kD; yellow asterisks) is higher compared with SDS-PAGE with the Laemmli buffer system (compare with Fig. 2). proCLE40 and its cleavage products are indicated by red and blue asterisks, respectively. C, SBTs cleave proCLE40 at the C terminus and at an internal site of the mature peptide. The peptide fraction (less than 10 kD) of the digest in B was analyzed by liquid chromatography-electrospray ionization-MS/MS. Precursor-derived cleavage products are shown for SBT1.4, SBT1.7, and the SBT4.13 digest.