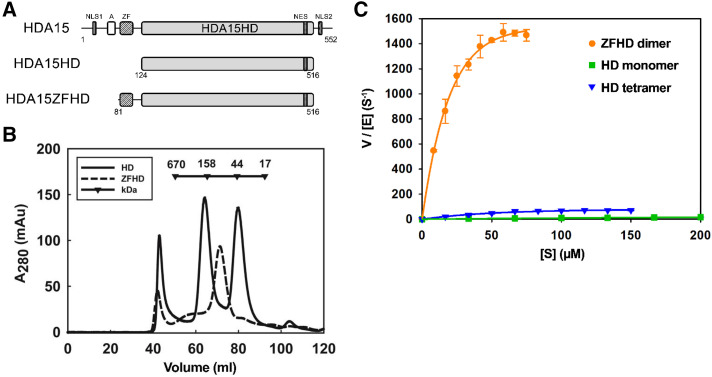
Figure 1.
The zinc finger domain enhances HDA15HD dimerization and enzymatic activity in vitro. A, Schematic structure of HDA15 protein domains. A, Asp-rich region; HD, histone deacetylase domain; NLS, nuclear localization signal; ZF, zinc finger domain. B, Size exclusion chromatography of the HDA15HD monomer (42 kD), tetramer (168 kD), and HDA15ZFHD dimer (94 kD). C, Enzyme kinetics of the HDA15HD monomer, tetramer and HDA15ZFHD dimer. The enzyme concentrations [E] of HD monomer, tetramer and HDA15ZFHD dimer were 75, 25, and 2.5 nm, respectively. V indicates the initial velocity (μm s−1). The values represent the mean (± sd) of three replicates.