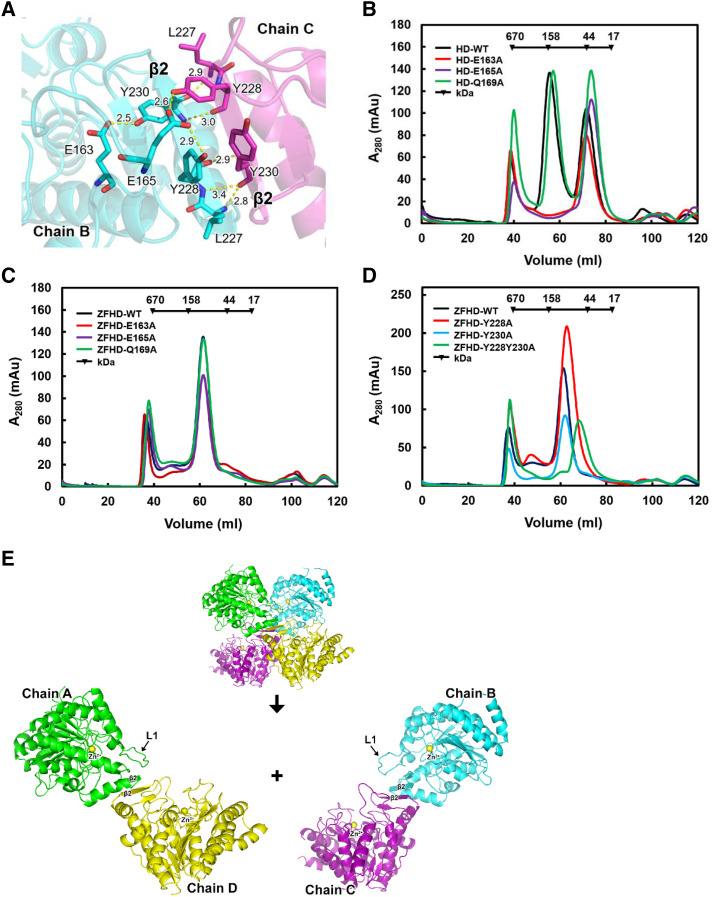
Figure 3.
The zinc finger domain enhances HDA15HD dimerization and enzymatic activity in vitro. A, Ribbon diagram representation of the crystal structure of the HDA15HD tetramer interface in L1 (E163 and E165) and β2 (Y228 and Y230). Hydrogen bonds are represented by yellow dotted lines. The number indicates the length of the hydrogen bond. B, Size exclusion chromatography of HDA15HD with mutations in L1 (E163, E165, and Q169). C, Size exclusion chromatography of HDA15ZFHD with mutations in L1 (E163, E165, and Q169). D, Size exclusion chromatography of HDA15ZFHD with mutations in β2 (Y228 and Y230). E, The HDA15HD tetramer is formed by two “head-to-head” dimers (chain A/D × chain B/C). WT, wild type.