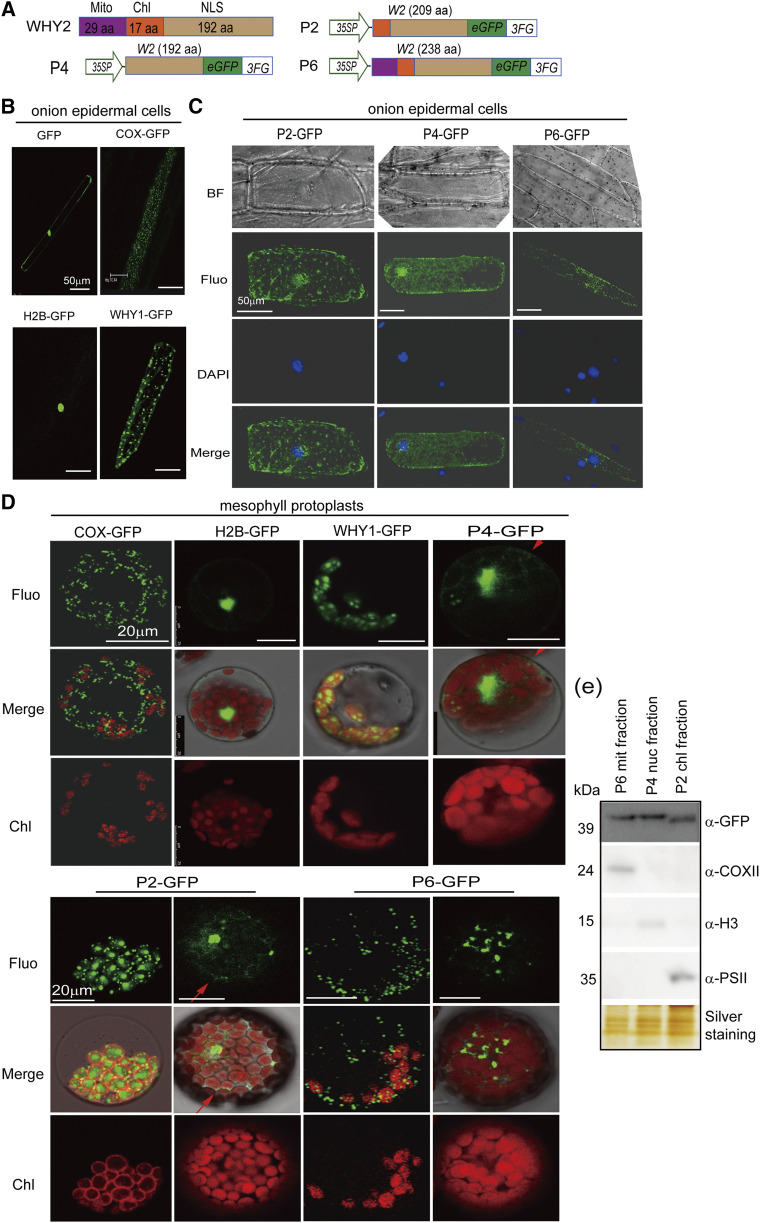
Figure 4.
Detection of WHY2 triple localization. A, Schematic of a series of WHY2 deletion constructs. P2: WHY2 with deleted mitochondrion transit peptide fused to GFP. P4: WHY2 with deleted plastid and mitochondrial transit peptide fused to GFP. P6: full-length WHY2 fused to GFP. Scale bars = 50 μm. B, Positive control plasmids (COX-GFP, H2B-GFP, WHY1-GFP) expressed in onion epidermal cells by gene gun biolistic assay. C, Observation of P2, P4, and P6 subcellular localization in onion epidermal cells by gene gun biolistic assay. BF, bright field; Fluo, Fluorescence; DAPI, 4′,6-diamino-phenylindole staining the nucleus; scale bars = 50 μm. D, Observation of P2, P4, and P6 subcellular localization in Arabidopsis mesophyll cells by protoplast transit assay. The plasmids of COX-GFP, H2B-GFP, and WHY1-GFP were used as positive controls. Scale bars = 20 μm. E, Western blot detection of WHY2 expression in the mitochondria (Mito) fractions isolated from P6, nuclear fraction isolated from P4, and chloroplast (Chl) fraction isolated from P2 using an antibody against GFP. Anti-PSII was used as a chloroplast protein control, anti-H3 as a nuclear protein control, and anti-COXII as a mitochondrial protein control. Silver staining of the protein gel was used to indicate loading.