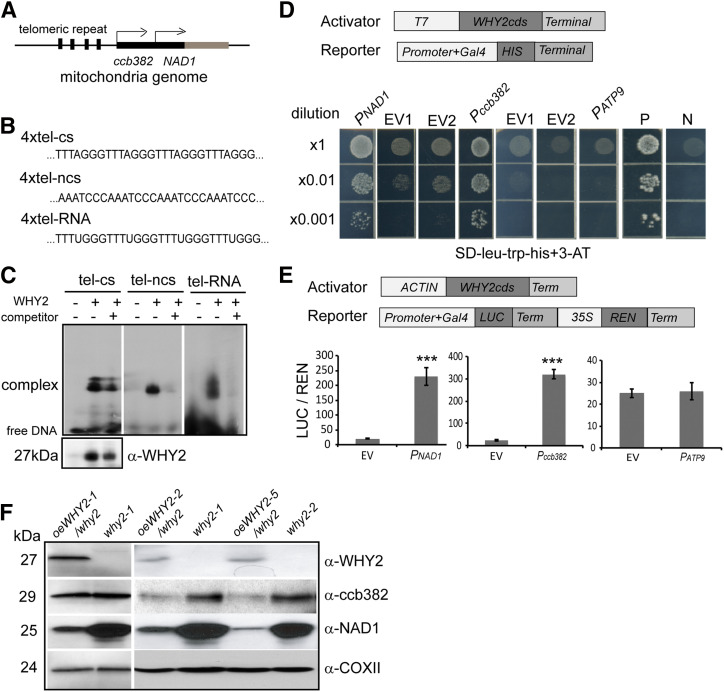
Figure 6.
WHY2 binds to the upstream region of NAD1 and ccb382 of the mitochondrial genome and promotes their expression. A, Diagram of the upstream regions of NAD1 and ccb382 genes. B, The sequences of the upstream regions of NAD1 and ccb382 genes. tel-cs, coding strand; tel-ncs, noncoding strand; tel-RNA, RNA sequence. C, EMSA. The tel-cs, tel-ncs, and tel-RNA fragments were used as the probe (labeled with biotin). Twentyfold excess competitor probe (without biotin) was added as a specificity control. The recombinant WHY2, expressed and isolated from E. coli, used in the reaction was detected by Western blot with anti-WHY2. D, Yeast one-hybrid assay results. The pNAD1, pccb382, and pATP9 fragments were inserted into the pHIS2 expression vector. The various dilutions of colonies on the selective medium showed the activation of expression by WHY2 of the HIS reporter gene driven by the indicated fragments in yeast. EV1, the plasmid GAD-WHY2 with the empty pHIS2; EV2, the empty plasmid GAD with promoter fragment fused HIS2 plasmid; they were cotransformed into the yeast strain AH109 as negative controls: P, positive, N, negative. E, Luciferase (LUC)/Renilase (REN) dual activation assay. Agrobacterium cells containing the vectors expressing WHY2-FLAG (ACTIN:WHY2-FLAG) and the Agrobacterium cells containing the vectors expressing fragments: LUC-REN were coinjected into Nicotiana benthamiana leaves. ATP9 promoter was used as a negative control. Shown are mean and SE of six biological replicates. Asterisks denote statistically significant differences from the empty vector, calculated using Student’s t test: *P < 0.05; **P < 0.01; and ***P < 0.001. F, Western blot detection of NAD1 and ccB382 protein levels in the oeWHY2 and why2 lines. COXII was used as a loading control.