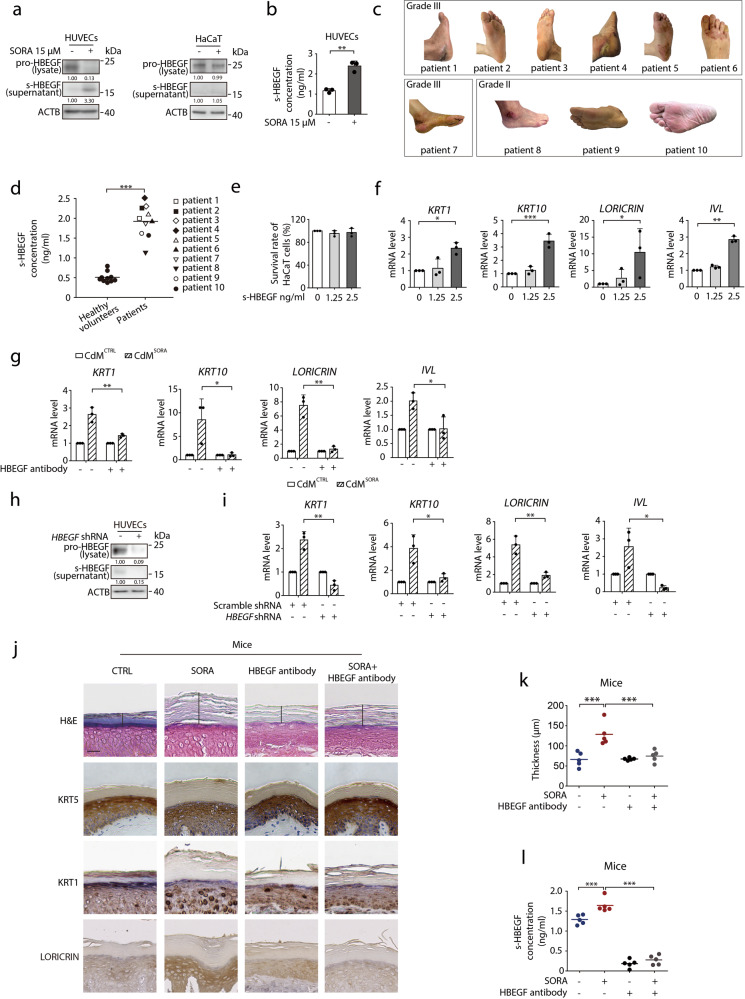
Fig. 2. s-HBEGF governs sorafenib-induced hyper-keratosis.
a HUVECs or HaCaT cells were exposed to 15 μM sorafenib for 24 h. The level of s-HBEGF in the supernatant or pro-HBEGF in total cell lysates was detected by western blot. b s-HBEGF concentrations in supernatants of HUVECs were measured by HBEGF peptide enzyme-linked immunosorbent assay (ELISA) (n = 3). c Representative photos of ten patients with HFSR of various grades. d s-HBEGF concentrations in the serum of ten healthy volunteers and ten patients were measured by ELISA. e HaCaT cells were treated with s-HBEGF for 72 h. Cell survival rate was detected by SRB assay (n = 3). f RT-qPCR analysis of KRT1, KRT10, LORICRIN and IVL in HaCaT cells treated with s-HBEGF recombinant protein for 24 h (n = 3). g HaCaT cells were treated with CdMCTRL or CdMSORA in the presence of HBEGF neutralization antibody for 24 h. The transcription levels of KRT1, KRT10, LORICRIN and IVL were detected by RT-qPCR (n = 3). h HUVECs were transfected with scramble shRNA or HBEGF shRNA via lentivirus. The level of s-HBEGF in the supernatant or pro-HBEGF in total cell lysates was detected by western blot. i CdMCTRL or CdMSORA was collected from HUVECs transfected with scramble shRNA or HBEGF shRNA. HaCaT cells were treated with CdMCTRL or CdMSORA for 24 h. The transcription levels of KRT1, KRT10, LORICRIN and IVL were detected by RT-qPCR (n = 3). j–l Mice were treated with HBEGF neutralization antibody (100 ng/mouse) twice a week by i.v. and/or sorafenib (100 mg/kg) daily by i.g. for 30 days (n = 5/group). j Representative H&E staining and KRT5, KRT1, LORICRIN immunohistochemistry staining were performed on the paws of mice. Scale bar, 50 μm. k Quantitative analysis of epidermal hyper-keratosis assessed by measuring the stratum corneum thickness (n = 5/group). l s-HBEGF concentrations in the serum of each mouse were measured by ELISA (n = 5/group). Densitometric values are shown as optical density after ACTB normalization using Image J. Horizontal bars in (d), (k) and (l) represent mean values. The results in (b), (e), (f), (g) and (i) are presented as the mean ± SD. Statistical analyses were performed using unpaired two-tailed Student’s t test in (b), (d), (g) and (i). Statistical analyses were performed using one-way ANOVA with Dunn’s post hoc test when comparing the levels of KRT1 and IVL and with LSD post hoc test when comparing the levels of KRT10 and LORICRIN in (f) and with LSD post hoc test in (k) and (i). *P < 0.05; **P < 0.01; ***P < 0.001. SORA sorafenib, CdM HUVECs conditional medium, CTRL control.