Abstract
Purpose
To report our 14-year experience with orbital exenteration and assess risk factors for poor prognosis by focusing on conjunctival melanoma.
Patients and method
A retrospective study was conducted in our tertiary care centre (Jules Gonin Eye Hospital, Lausanne, Switzerland) between 2003 and 2017. Inclusion criteria were patients aged ≥18 years with a follow-up >12 months, without metastatic spread at the time of surgery. Data recorded were age, gender, tumour histology, surgical technique, postoperative complications, surgical margin status, local recurrence, postoperative radiation beam therapy and metastatic status.
Results
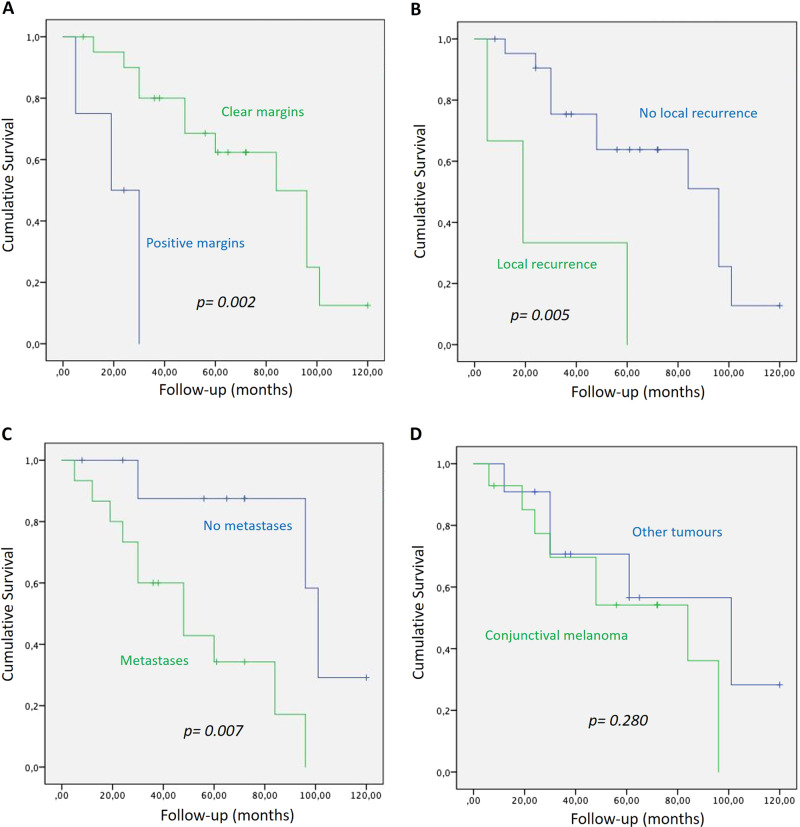
Twenty-five patients with a mean age of 63.2 years (38–92) were included. Conjunctival melanoma was the most frequently identified tumour (n = 14, 56%) followed by conjunctival squamous cell carcinoma (n = 4, 16%), sebaceous carcinoma (n = 3, 12%), choroidal melanoma (n = 2, 8%) and basal cell carcinoma (n = 2, 8%). Eighteen tumours (72%) originated from the conjunctival tissue. Clear surgical margins were achieved in 21 (84%) patients. Fourteen (56%) patients experienced distant metastases and died from metastatic spread after a mean follow-up of 52.3 months (6–120). The 1-, 3- and 5-year overall survival (OS) was 96%, 72% and 60%, respectively. In the univariate analysis, positive surgical margins, local recurrence and metachronous metastases were associated with a decreased OS (p = 0.002, p = 0.005 and p = 0.007, respectively). In the multivariate analysis, positive surgical margins and metachronous metastases were also associated with a decreased OS (p = 0.02 and p = 0.042, respectively). Conjunctival melanoma was not associated with a poorer prognosis (p = 0.280).
Conclusion
Free surgical margins are needed to increase OS. To achieve clearer surgical margins, neoadjuvant targeted therapies/immunotherapies may be considered.
Subject terms: Eye cancer, Conjunctival diseases
Introduction
Orbital exenteration is a radical and disfiguring surgical procedure. It consists of removing the entire orbital contents with a subperiosteal dissection. One can oppose eyelid sparing, total and enlarged orbital exenteration [1, 2]. Orbital exenteration with eyelid sparing is to remove the entire orbital content without the eyelids and is therefore best suited for posterior orbital tumours. Total exenteration involves the removal of the entire orbital content, including the eyelids. Extended orbital exenteration involves the removal of the entire orbital content together with the paranasal sinus and/or bony orbit. Orbital exenteration is indicated for tumours originating in the eyelids, the eye, the orbit or the paranasal sinuses [2]. The recent literature has highlighted a highly variable 5-year overall survival (OS) rate following orbital exenteration (26–92%) [2–8]. This variability depends on the type of tumours taken into account in each study. For example, eyelid basal cell carcinoma (BCC) is the most common treated tumour reported in the literature [2–8] while conjunctival melanoma is rarely found [9]. The study duration is also of prime interest because regional and distant metastases can occur years after obtaining the local control of the disease [10]. Data on orbital exenteration for conjunctival melanoma are limited [10, 11]. Our centre is a tertiary care centre specialised in ocular oncology. We are more often faced with conjunctival and uveal tumours than with malignant eyelid tumours. So far, no study has compared the OS of patients exenterated for conjunctival melanoma to that of patients exenterated for other malignancies. The aim of this study was to describe patient follow-up after orbital exenteration over a 14-year period and to assess risk factors for a poorer prognosis by focusing on conjunctival melanoma.
Patients and method
This retrospective monocentric case series was conducted between December 2003 and December 2017 in our tertiary care centre (Jules Gonin Eye Hospital, Lausanne, Switzerland) specialised in ocular oncology. Patients aged over 18 years who had undergone orbital exenteration for malignancies with a follow-up of at least 12 months were included. Patients with lymph node involvement or distant metastases at the time of surgery were excluded. The following data were recorded: age, sex, previous treatments, type of orbital exenteration, operative and postoperative complications, tumour histology, margin status, postoperative radiation beam therapy, systemic metastatic status and local recurrence. The local recurrence was assessed by a clinical examination of the sockets reconstructed with spontaneous granulation. Orbital computed tomography was performed every 6 months for sockets reconstructed with lid or regional flaps. Orbital MRI was avoided due to the artefacts of the orbital implants used for episthesis retention. The metastatic status was assessed every 6 months by 18F-FDG-PET/CT or contrast-enhanced cervical, chest, abdominal and pelvis CT scan.
No approval from an ethics committee was needed because it was a retrospective, non-invasive study conducted in accordance with the tenets of the Declaration of Helsinki and the French Jarde law (2016 version).
Data were analysed using SPSS software (Chicago, Illinois). Descriptive statistics are presented as counts and percentages for categorical variables and as a mean ± standard deviation for continuous variables. Analyses were carried out using Fisher exact and Mann–Whitney tests to compare qualitative and quantitative data, respectively. OS was defined as the time from orbital exenteration to death or the last follow-up. OS was assessed using a Kaplan Meier analysis. A log-rank test was used to compare survival distribution between two groups. A multivariate analysis was carried out using a Cox proportional Hazards model. A p value < 0.05 was considered statistically significant.
Results
Twenty-five patients (9 men, 16 women) were included in the study. Patient characteristics and surgical features are shown in Table 1. Patient mean age was 63.2 (38–92) years. Conjunctival melanoma was the most common tumour (n = 14, 56%) followed by squamous cell carcinoma (n = 4, 16%), lid sebaceous carcinoma (n = 3, 12%), extra-scleral choroidal melanoma (n = 2, 8%) and BCC (n = 2, 8%). Eighteen (72%) tumours originated from the conjunctival tissue. Sixteen (64%) and 11 (44%) patients had undergone previous surgeries or radiation beam therapy before orbital exenteration, respectively. Only seven (28%) patients had directly undergone orbital exenteration. Total exenteration was the most commonly used surgical technique (n = 22, 88%). The most commonly used technique for socket reconstruction was healing by secondary intention (n = 22, 88%). No operative complications were recorded. Regarding postoperative complications, ethmoidal fistula was the most commonly found (n = 9, 36%). Three months after ablative surgery, one (4%) patient developed zygomatic infectious osteitis caused by orbital endosseous implant placement, and after implant ablation, the outcome was favourable. Positive surgical margins were identified in four (16%) patients. Among them, three (12%) patients had received adjuvant conventional orbital radiation therapy whereas one (4%) refused this treatment.
Table 1.
Patients characteristics, and surgical and pathological features.
| Number (%) | Mean (Range, SD) | |
|---|---|---|
| Patients | 25 (100) | |
| Men/women | 9 (36)/16 (64) | |
| Age (years) | 63.2 (38–92, 16.4) | |
| Surgical indications: | ||
| Conjunctival melanoma | 14 (56) | |
| Conjunctival squamous cell carcinoma | 4 (16) | |
| Lid sebaceous carcinoma | 3 (12) | |
| Choroidal melanoma | 2 (8) | |
| Basal cell carcinoma | 2 (8) | |
| Tumour location: | ||
| Conjunctival | 18 (72) | |
| Eyelid | 5 (20) | |
| Intraocular | 2 (8) | |
| Previous treatments receiveda | ||
| Surgery | 16 (64) | |
| Proton beam therapy | 9 (36) | |
| Conventional radiation beam therapy | 2 (8) | |
| Mitomycin C | 5 (20) | |
| Brachytherapy | 9 (36) | |
| None | 7 (28) | |
| Surgical technique | ||
| Lid-sparing orbital exenteration | 1 (4) | |
| Non-lid-sparing orbital exenteration | 22 (88) | |
| Enlarged orbital exenteration | 2 (8) | |
| Reconstruction of the exenterated socket: | ||
| Spontaneous granulation | 22 (88) | |
| Lid flaps | 1 (4) | |
| Regional flaps | 2 (8) | |
| Postoperative complications | ||
| Ethmoidal fistula | 9 (36) | |
| Osteitis | 1 (4) | |
| None | 15 (60) | |
| Surgical Margin status | ||
| Clear | 21 (84) | |
| Invaded | 4 (16) | |
aResults >100% because multiple treatments were received
SD standard deviation
Follow-up data are shown in Table 2. The mean follow-up duration was 52.3 (12–120) months. A local recurrence was found in three (12%) patients. Among them, only one (4%) patient had previously received postoperative radiation beam therapy. All patients who experienced a local recurrence had received adjuvant radiation beam therapy and no recurrence was noted. Fourteen (56%) patients experienced regional or systemic metastases during the follow-up period. All these patients died due to systemic metastatic spread. The 1-, 3- and 5-year OS was 96%, 72% and 60%, respectively (Fig. 1). The univariate analysis of the OS showed that positive surgical margins (p = 0.002), a local recurrence (p = 0.005) and metachronous metastases (p = 0.007) were associated with a poorer prognosis (Fig. 2a, b, c), while conjunctival melanoma was not (p = 0.280) (Fig. 2d). The multivariate analysis showed that positive surgical margins and metachronous metastases were also significantly associated with a decreased OS (p = 0.02 and p = 0.042, respectively).
Table 2.
Follow-up and overall survival (OS).
| Number (%) | Mean (Range, SD) | |
|---|---|---|
| Local recurrence | ||
| Yes | 3 (12) | |
| No | 22 (88) | |
| Adjuvant radiation beam therapy: | ||
| Yes | 4 (16) | |
| No | 21 (84) | |
| Metachronous metastases | ||
| Yes | 14 (56) | |
| No | 11 (44) | |
| Follow-up (months) | 52.3 (12–120, 31.5) | |
| OS at 1 year | 24/25 (96) | |
| OS at 3 years | 18/25 (72) | |
| OS at 5 years | 15/25 (60) | |
Fig. 1.
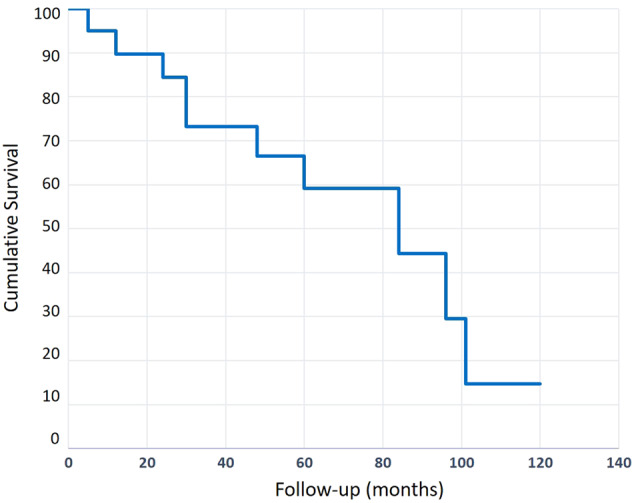
Kaplan Meier cumulative survival analysis for all patients.
Fig. 2. Kaplan Meier cumulative survival analysis for each group.
a OS of patients with positive surgical margins versus clear surgical margins. b OS of patients with local recurrence versus no local recurrence. c OS of patients with metachronous metastases versus no distant metastases. d OS of patients with conjunctival melanoma versus other tumours.
Discussion
Orbital exenteration is a radical and disfiguring surgical procedure mainly performed in case of orbital cancers. BCC is the most common tumour reported in the current literature [1, 6, 12–14]. In our study, however, conjunctival melanoma was the most prevalent tumour (n = 14/25, 56%). This difference could be explained by the fact that our centre is a tertiary care centre specialised in ocular oncology, especially in conjunctival and uveal tumours. In our study, only two patients were treated for eyelid BCC. Since 2012, advanced BCC are mainly treated with anti-SMO targeted therapies [15, 16]. Sagiv et al. have stressed the fact that, since vismodegib has been approved by the FDA, performing orbital exenteration for locally advanced BCC has been significatively reduced [17]. In our centre, anti-SMO targeted therapies are usually prescribed, and this could explain the small number of BCC patients who underwent orbital exenteration in our study.
Total orbital exenteration was the most common orbital exenteration technique used in our centre. Epithelialization of the exenterated socket was often achieved with healing by secondary intention. Some authors have advocated the use of the lid-sparing orbital exenteration technique with favourable outcomes. According to our experience, two major limitations should be emphasised with this technique. First, lid-sparing surgery does not result in enough concave socket shape and thus can lead to episthesis retention failure. In addition, this technique reduces pathological surgical margins in case of malignancies located anteriorly (conjunctival or lid tumours). Surprisingly, lid-sparing exenteration has been used by Shields et al. for the management of 22 out of 24 conjunctival malignancies [11]. Two (8%) patients in our study had undergone enlarged orbital exenteration up to the ethmoidal sinus. No operative complications were recorded over the study period. Cerebrospinal fluid leakage is the leading life-threatening complication with an incidence ranging between 0 and 13% [8, 18]. Other known operative complications are sinus fistula and orbital haemorrhage [1] but they were not found in our study.
Ethmoidal fistula was the most common postoperative complication identified in our study (n = 9/25, 36%). No patients reported related symptoms requiring surgical closure of the fistula. Our findings are in accordance with previous studies in which ethmoidal fistula has been reported in up to 50% of cases [2, 19–21]. Postoperative infection has been identified in 0–43% of cases according to studies [2, 20, 21]. In our study, one (4%) patient developed postoperative osteitis three months after implant placement and required its surgical removal and the use of systemic antibiotics with favourable outcomes. Death and postoperative cardiovascular disorders are exceptional but have already been reported [22, 23]. None occurred in our study.
Free surgical margins were achieved in 21 out of 25 patients (84%). This result is in line with previous studies (in 42.5% [6] to 97% [18] of cases).
In our study, the 1-, 3- and 5-year OS was 96%, 72% and 60%, respectively. These results are in accordance with previous studies. In the current literature, the 1-year OS ranges between 50.5% [24] and 97% [2] and the 5-year OS ranges between 37% [7] and 92% [8]. Kuo et al. have reported a 5-year OS of 92% and they have advocated the need for prompt and aggressive surgical treatment [8]. However, to date, no study has shown an OS improvement following orbital exenteration. Recent retrospective studies have reported favourable outcomes without performing orbital exenteration when managing small (<T3 according to the 7th TNM classification) epithelial lacrimal gland cancers [25, 26]. A recent ENT literature review has also failed to demonstrate the superiority of orbital exenteration over conservative surgery for the management of sinus malignancies invading the orbital contents [27].
The risk factors associated with a shorter OS are also debated in the literature. Comparing the literature is challenging due to the scarcity of orbital exenteration and the heterogeneity of inclusion criteria used in the studies. In our study, the tumour histology was not associated with a poorer prognosis, and given the aggressiveness of conjunctival melanoma, we assumed that it would have been associated with a poorer systemic prognosis. In their study, Wong et al. have shown a better prognosis for patients with BCC compared with other tumours [6]. Surprisingly, these results have not been confirmed by Rahman et al. [28]. and Gerring et al. [13].
In our study, positive surgical margins were associated with a shorter OS according to the log-rank test (p = 0.002). These results are consistent with those published by Gerring et al. [13], although other authors have not been able to show any OS improvement despite the presence of clear surgical margins [4, 7, 12, 28]. Another important factor known to affect OS is the presence of synchronous micro-metastases that are suspected to be already present at the time of orbital exenteration. In accordance with Aryasit et al. [29], a local recurrence was associated with a decreased OS in our study, as well as the presence of metachronous metastases.
Our study has some limitations. It is a small retrospective study. However, orbital exenteration is a rare procedure and patients with synchronous metastases at the time of diagnosis were excluded from our study. We did not show any significant correlation between the OS and conjunctival melanoma. However, the mean follow-up duration was 52.3 (12–120) months so that the long-term survival should be interpreted with caution. In addition, it has been shown that conjunctival melanomas could harbour BRAF, RAS or NF-1 mutations [30] and the prescription of systemic targeted therapies (anti-BRAF) or immunotherapies could have influenced our results. However, to the best of our knowledge, none of our patients had been treated with these therapies. Prescribing these drugs as neoadjuvants may help surgeons to achieve free surgical margins and may reduce or eliminate micro-metastases. Also immunotherapy (anti-PD-1) could be a valid neoadjuvant therapy, since it has recently shown encouraging results in locally advanced and metastatic conjunctival melanoma [31, 32].
In conclusion, conjunctival melanoma was the most frequently identified tumour in our study. The 1-, 3- and 5-year OS was 96%, 72% and 60%, respectively. Positive surgical margins (p = 0.02) and metachronous metastases (p = 0.042) were associated with a decreased OS in the multivariate analysis. This study stresses the need for free surgical margins to increase OS. Neoadjuvant targeted therapies or immunotherapies could help to achieve clearer surgical margins. Further studies are needed to confirm our findings.
Summary
What was known before
Orbital exenteration is radical and disfiguring surgical procedure basal cell carcinoma is the most common tumour encountered.
Prognosis factors are debated.
What this study adds
Conjunctival melanoma is not associated with a poorer prognosis.
This study stresses the need for free surgical margins to increase overall survival neoadjuvant targeted therapies or immunotherapies could help to achieve clearer surgical margins.
Acknowledgements
Ms Pallaz for her help to collect all the data.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang Z, Ho S, Yin V, Varas G, Rajak S, Dolman PJ, et al. Multicentred international review of orbital exenteration and reconstruction in oculoplastic and orbit practice. Br J Ophthalmol. 2018;102:654–8. doi: 10.1136/bjophthalmol-2017-310681. [DOI] [PubMed] [Google Scholar]
- 2.Kiratli H, Koç İ. Orbital exenteration: Institutional review of evolving trends in indications and rehabilitation techniques. Orbit. 2018;37:179–86. doi: 10.1080/01676830.2017.1383466. [DOI] [PubMed] [Google Scholar]
- 3.Bartley GB, Garrity JA, Waller RR, Henderson JW, Ilstrup DM. Orbital exenteration at the Mayo Clinic. 1967-1986. Ophthalmology. 1989;96:468–73. doi: 10.1016/S0161-6420(89)32872-7. [DOI] [PubMed] [Google Scholar]
- 4.Mouriaux F, Martinot V, Pellerin P, Patenotre P, Rouland JF, Constantinides G. Survival after malignant tumors of the orbit and periorbit treated by exenteration. Acta Ophthalmol Scand. 1999;77:326–30. doi: 10.1034/j.1600-0420.1999.770316.x. [DOI] [PubMed] [Google Scholar]
- 5.Mohr C, Esser J. Orbital exenteration: surgical and reconstructive strategies. Graefes Arch Clin Exp Ophthalmol. 1997;235:288–95. doi: 10.1007/BF01739638. [DOI] [PubMed] [Google Scholar]
- 6.Wong JCL, Thampy R, Cook A. Life expectancy following orbital exenteration. Br J Ophthalmol. 2015;99:1–4. doi: 10.1136/bjophthalmol-2013-304436. [DOI] [PubMed] [Google Scholar]
- 7.Nagendran ST, Lee NG, Fay A, Lefebvre DR, Sutula FC, Freitag SK. Orbital exenteration: the 10-year Massachusetts Eye and Ear Infirmary experience. Orbit. 2016;35:199–206. doi: 10.1080/01676830.2016.1176210. [DOI] [PubMed] [Google Scholar]
- 8.Kuo C-H, Gao K, Clifford A, Shannon K, Clark J. Orbital exenterations: an 18-year experience from a single head and neck unit. ANZ J Surg. 2011;81:326–30. doi: 10.1111/j.1445-2197.2010.05592.x. [DOI] [PubMed] [Google Scholar]
- 9.Kao A, Afshar A, Bloomer M, Damato B. Management of primary acquired melanosis, nevus, and conjunctival melanoma. Cancer Control J Moffitt Cancer Cent. 2016;23:117–25. doi: 10.1177/107327481602300205. [DOI] [PubMed] [Google Scholar]
- 10.Paridaens AD, McCartney AC, Minassian DC, Hungerford JL. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78:520–8. doi: 10.1136/bjo.78.7.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shields JA, Shields CL, Demirci H, Honavar SG, Singh AD. Experience with eyelid-sparing orbital exenteration: the 2000 Tullos O. Coston Lecture. Ophthal Plast Reconstr Surg. 2001;17:355–61. doi: 10.1097/00002341-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman GR, Jefferson ND, Reid CBA, Eisenberg RL. Orbital exenteration to manage infiltrative sinonasal, orbital adnexal, and cutaneous malignancies provides acceptable survival outcomes: an institutional review, literature review, and meta-analysis. J Oral Maxillofac Surg. 2016;74:631–43. doi: 10.1016/j.joms.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Gerring RC, Ott CT, Curry JM, Sargi ZB, Wester ST. Orbital exenteration for advanced periorbital non-melanoma skin cancer: prognostic factors and survival. Eye. 2017;31:379–88. doi: 10.1038/eye.2016.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langlois B, Jacomet P-V, Putterman M, Morax S, Galatoire O. Evaluation of reconstructive techniques after orbital exenteration in 56 cases. J Fr Ophtalmol. 2012;35:667–77. doi: 10.1016/j.jfo.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Wong KY, Fife K, Lear JT, Price RD, Durrani AJ. Vismodegib for locally advanced periocular and orbital basal cell carcinoma: a review of 15 consecutive cases. Plast Reconstr Surg Glob Open. 2017;5:e1424. doi: 10.1097/GOX.0000000000001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sagiv O, Nagarajan P, Ferrarotto R, Kandl TJ, Thakar SD, Glisson BS, et al. Ocular preservation with neoadjuvant vismodegib in patients with locally advanced periocular basal cell carcinoma. Br J Ophthalmol. 2019;103:775–80. [DOI] [PubMed]
- 17.Sagiv O, Ding S, Ferrarotto R, Glisson B, Altan M, Johnson F, et al. Impact of food and drug administration approval of vismodegib on prevalence of orbital exenteration as a necessary surgical treatment for locally advanced periocular basal cell carcinoma. Ophthal Plast Reconstr Surg. 2019;35:350–3. [DOI] [PubMed]
- 18.Nassab RS, Thomas SS, Murray D. Orbital exenteration for advanced periorbital skin cancers: 20 years experience. J Plast Reconstr Aesthetic Surg. 2007;60:1103–9. doi: 10.1016/j.bjps.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Ali MJ, Pujari A, Dave TV, Kaliki S, Naik MN. Clinicopathological profile of orbital exenteration: 14 years of experience from a tertiary eye care center in South India. Int Ophthalmol. 2016;36:253–8. doi: 10.1007/s10792-015-0111-5. [DOI] [PubMed] [Google Scholar]
- 20.Soysal HG. Orbital exenteration: a 10-year experience of a general oncology hospital. Orbit. 2010;29:135–9. doi: 10.3109/01676830903342252. [DOI] [PubMed] [Google Scholar]
- 21.Taylor A, Roberts F, Kemp EG. Orbital exenteration—a retrospective study over an 11 year period analyzing all cases from a single unit. Orbit. 2006;25:185–93. doi: 10.1080/01676830600575584. [DOI] [PubMed] [Google Scholar]
- 22.Kato JM, Fonseca FL, da, Matayoshi S. Survival following orbital exenteration at a tertiary brazilian hospital. Rev Col Bras Cir. 2016;43:42–7. doi: 10.1590/0100-69912016001009. [DOI] [PubMed] [Google Scholar]
- 23.Roche P, Timon C. Orbital exenteration in periorbital malignancies. Surg J R Coll Surg. 2012;10:189–93. doi: 10.1016/j.surge.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Karabekmez FE, Selimoglu MN, Duymaz A, Karamese MS, Keskin M, Savaci N. Management of neglected periorbital squamous cell carcinoma requiring orbital exenteration. J Craniofac Surg. 2014;25:729–34. doi: 10.1097/SCS.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 25.Woo KI, Sagiv O, Han J, Frank SJ, Kim Y-D, Esmaeli B. Eye-preserving surgery followed by adjuvant radiotherapy for lacrimal gland carcinoma: outcomes in 37 patients. Ophthal Plast Reconstr Surg. 2018;34:570–4. [DOI] [PubMed]
- 26.Rose GE, Gore SK, Plowman NP. Cranio-orbital resection does not appear to improve survival of patients with lacrimal gland carcinoma. Ophthal Plast Reconstr Surg. 2019;35:77–84. [DOI] [PubMed]
- 27.Reyes C, Mason E, Solares CA, Bush C, Carrau R. To preserve or not to preserve the orbit in paranasal sinus neoplasms: a meta-analysis. J Neurol Surg Part B Skull Base. 2015;76:122–8. doi: 10.1055/s-0034-1390403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman I, Cook AE, Leatherbarrow B. Orbital exenteration: a 13 year Manchester experience. Br J Ophthalmol. 2005;89:1335–40. doi: 10.1136/bjo.2004.062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aryasit O, Preechawai P, Hirunpat C, Horatanaruang O, Singha P. Factors related to survival outcomes following orbital exenteration: a retrospective, comparative, case series. BMC Ophthalmol. 2018;18:186. doi: 10.1186/s12886-018-0850-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scholz SL, Cosgarea I, Süßkind D, Murali R, Möller I, Reis H, et al. NF1 mutations in conjunctival melanoma. Br J Cancer. 2018;118:1243–7. doi: 10.1038/s41416-018-0046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sagiv O, Thakar SD, Kandl TJ, Ford J, Sniegowski MC, Hwu W-J, et al. Immunotherapy with programmed cell death 1 inhibitors for 5 patients with conjunctival melanoma. JAMA Ophthalmol. 2018;136:1236–41. doi: 10.1001/jamaophthalmol.2018.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finger PT, Pavlick AC. Checkpoint inhibition immunotherapy for advanced local and systemic conjunctival melanoma: a clinical case series. J Immunother Cancer. 2019;7:83. doi: 10.1186/s40425-019-0555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]