Figure 4 |. Therapeutic approaches to targeting the BANGs in cancer.
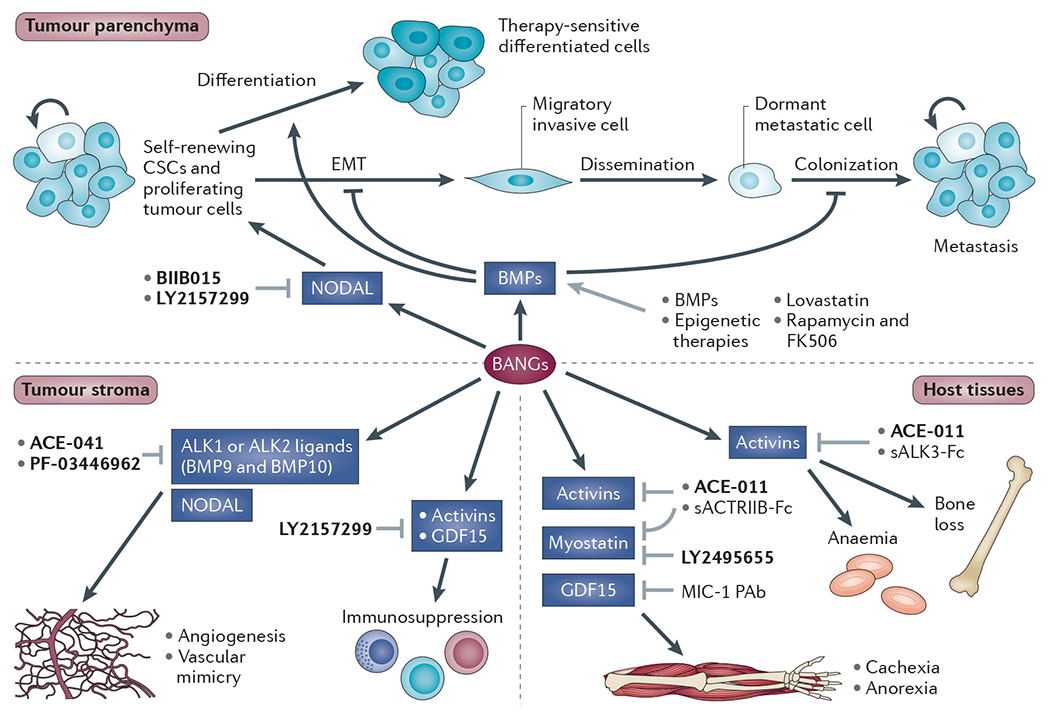
Dysregulation of bone morphogenetic proteins (BMPs), activins, NODAL, and growth and differentiation factors (GDFs) (BANGs) can have deleterious effects on the tumour parenchyma, the tumour stroma and on host tissues that are not directly involved in the tumorigenic process. Broadly, most therapeutic strategies to date have been aimed at either enhancing BMP activity or antagonizing BANGs of the activin and NODAL superfamily. For more details of therapeutic agents under clinical development (shown in bold) see TABLE 2. The other therapeutic agents shown are still at the preclinical development stage. Of these, the BMP ligands (some of which are already US Food and Drug Administration (FDA)-approved for fracture healing and lumbar fusion) have been used to induce cancer stem cell (CSC) differentiation and to restore response to chemotherapeutics50,107. Genetic knockdown of enhancer of zeste homolog 2 (EZH2), a component of the Polycomb repressor complex that is highly expressed in many tumours, restored tumour suppressive BMP responses in glioma cells, suggesting promise for epigenetic therapies that can reverse gene silencing51. Interestingly, some drugs developed in other contexts can activate BMP signalling. Lovastatin, a cholesterol-lowering agent, restored response to 5-fluorouracil by reactivating epigenetically silenced BMP2 in colorectal and gastric cancers111, and the immunosuppressive agents FK506 and rapamycin can also activate BMP signalling112,113. Soluble activin receptor-like kinase 3-Fc (sALK3-Fc) and soluble activin receptor IIB-Fc (sACTRIIB-Fc) are ligand traps that have shown therapeutic promise as BANG antagonists in preclinical studies but that have not been taken into the clinic94,101. The grey arrows indicate interventions that may affect BANG signalling in cancer and cancer-associated processes. MIC-1 PAb, polyclonal antibody to GDF15.
