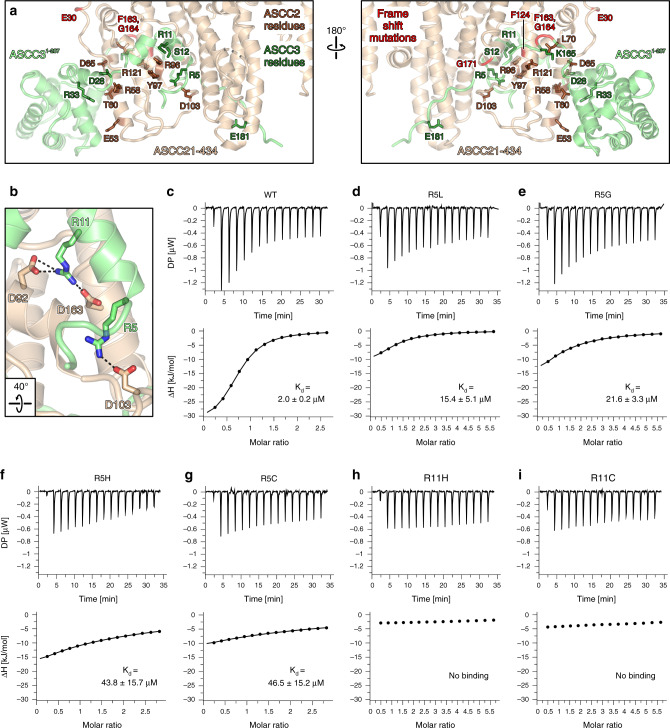
Fig. 4. Interface residues in ASCC2 and ASCC3 affected by somatic cancer mutations.
a Orthogonal views on the ASCC21–434-ASCC31–207 structure, highlighting interface residues and frameshift positions affected by somatic cancer mutations. ASCC21–434, beige; ASCC31–207, lime green; ASCC2 residues, brown sticks; ASCC3 residues, dark green sticks; first positions affected by frameshift mutations, red. Orientations of the panels as in Fig. 1d,f. b Details of the interaction networks involving cancer-related residues D103 of ASCC2, as well as R5 and R11 of ASCC3. Dashed lines, salt bridges. Rotation symbol, view relative to Fig. 1d,f, left. c–i ITC runs monitoring the interaction of ASCC21–434 with the indicated ASCC31–22 peptide variants. Deduced Kd values are listed as means ± SD for runs for which affinities could be quantified.