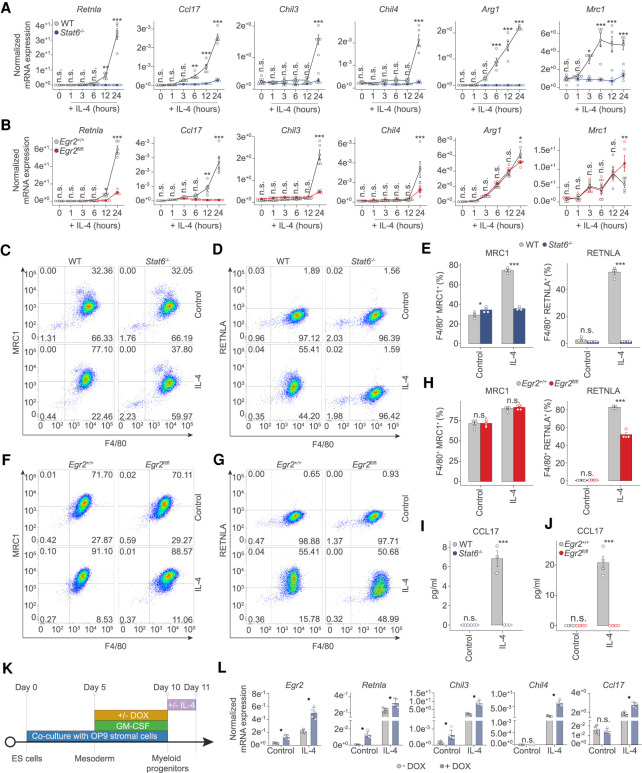
Figure 3.
Both STAT6 and EGR2 are required to regulate gene expression during alternative macrophage polarization. (A) RT-qPCR measurements on the indicated marker genes of alternative macrophage polarization from WT and Stat6−/− macrophages over the indicated time course of IL-4 treatment. The level of mRNA is normalized to the expression of Ppia. Experiments were repeated five times, and significant changes between groups were calculated by two-way analysis of variance (ANOVA). (B) RT-qPCR measurements on the indicated marker genes of alternative macrophage polarization from WT and Egr2fl/fl macrophages over the indicated time course of IL-4 treatment. The level of mRNA is normalized to the expression of Ppia. Experiments were repeated five times, and significant changes between groups were calculated by two-way analysis of variance (ANOVA). (C) Representative FACS plot of MRC1 (CD206) and ADGRE1 (F4/80) expression in control and IL-4 polarized macrophages from WT and Stat6−/− animals. (D) Representative FACS plot of RETNLA and ADGRE1 (F4/80) expression in control and IL-4 polarized macrophages from WT and Stat6−/− macrophages. (E) Percentages of MRC1 (CD206), ADGRE1 (F4/80) and RETNLA, ADGRE1 (F4/80) double-positive macrophages in control and IL-4 treated conditions from WT and Stat6−/− macrophages. Experiments were repeated three times, and significant changes between groups were calculated by two-way analysis of variance (ANOVA). (F) Representative FACS plot of MRC1 (CD206) and ADGRE1 (F4/80) expression in control and IL-4 polarized macrophages from Egr2+/+ and Egr2fl/fl animals. (G) Representative FACS plot of RETNLA and ADGRE1 (F4/80) expression in control and IL-4 polarized macrophages from Egr2+/+ and Egr2fl/fl animals. (H) Percentages of MRC1 (CD206), ADGRE1 (F4/80) and RETNLA, ADGRE1 (F4/80) double-positive macrophages in control and IL-4 treated conditions from Egr2+/+ and Egr2fl/fl animals. Experiments were repeated three times, and significant changes between groups were calculated by two-way analysis of variance (ANOVA). (I,J) CCL17 levels determined by ELISA in control and IL-4-polarized WT, Stat6−/− and Egr2+/+, Egr2fl/fl macrophages. Experiments were performed three times for the Stat6−/−, while four times for the Egr2fl/fl conditions, and significant changes were identified by two-way analysis of variance (ANOVA). (K) Experimental scheme of the gain of function experiments using embryonic stem cells (ES) differentiated towards the myeloid lineage in coculture with OP9 stromal cells, in the presence of GM-CSF. Doxycycline (DOX) was used to induce Egr2 levels at the indicated time before IL-4 treatment (24-h exposure). Cells were harvested for gene expression analysis at day 11. (L) RT-qPCR measurements of the indicated genes from embryonic cell differentiated myeloid cells harboring a doxycycline (DOX)-inducible EGR2 expressing construct. Cells were polarized with IL-4 (24 h) or left untreated (control). The level of mRNA is normalized to the expression of Ppia. Experiments were repeated five times and significant changes were identified by unpaired t-test at P < 0.05.