Fig. 2. Parallel fabrication of the theragrippers and their in vitro drug loading and release characteristics.
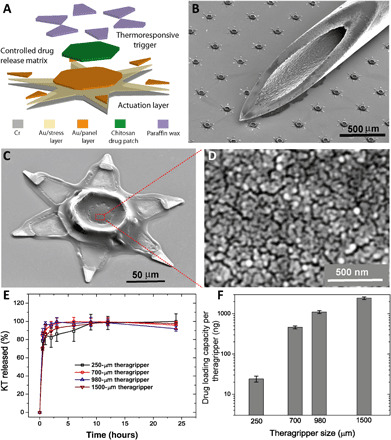
(A) Functional block diagram illustrating the microfabrication steps for an array of theragrippers, showing the actuation layer, drug-eluting layer, and the thermoresponsive trigger. (B) SEM image showing theragrippers next to the tip of a 22-gauge hypodermic needle. The theragrippers are small enough to pass safely through the GI tract without causing any gastric obstruction. (C) SEM image showing a single 250 μm, as fabricated theragripper with the drug-encapsulated chitosan patch at the center and the paraffin wax trigger layer on the hinges. (D) High-resolution SEM image showing the surface morphology of the chitosan patch at the center of the theragripper. The patch has pores less than 100 nm in size. (E) Release characteristics of ketorolac (KT) from theragrippers of four different sizes. (F) Plot showing the relative scaling of the drug loading capacity of theragrippers of different sizes. The entire loaded drug gets released over a period of 24 hours. While the 250-μm theragrippers were used for our in vivo experiments in rats, larger 1.5-mm theragrippers can be loaded with about 100 times more drug, for use in larger animal models and humans.
