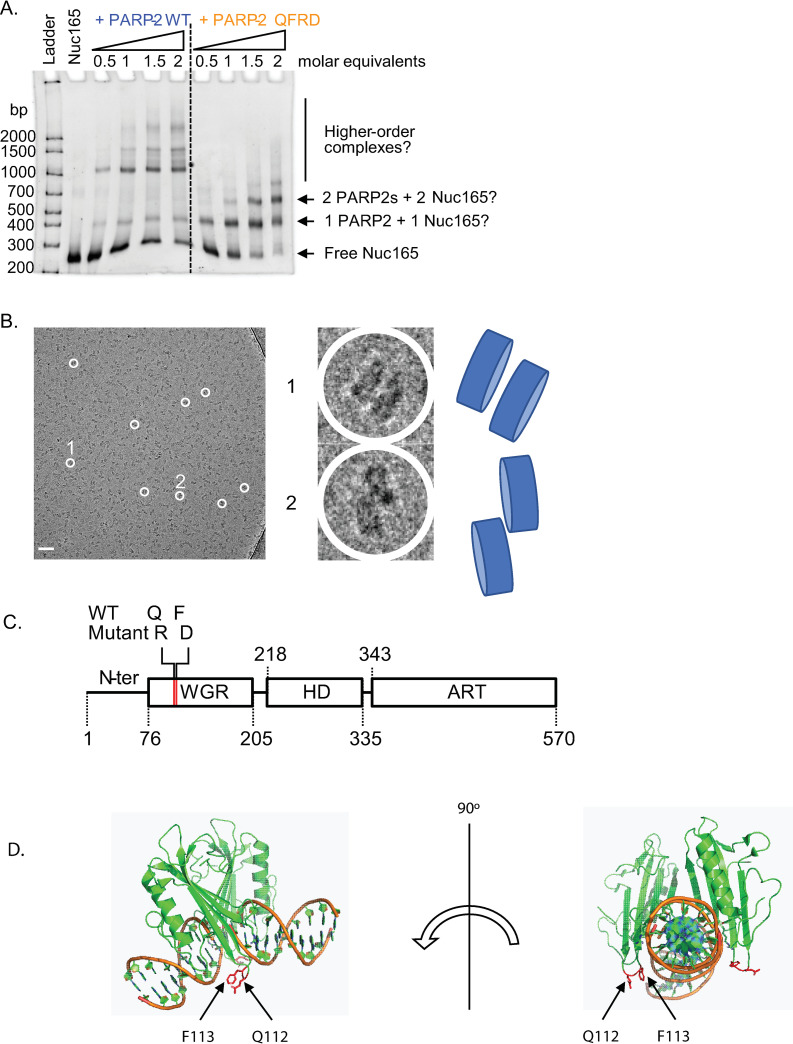
Fig 1. A double point mutant of PARP2 forms a homogeneous complex with Nuc165, suitable for cryo-EM.
A: Electrophoretic mobility shift assay of Nuc165 (1.4 μM) with increasing molar ratios of PARP2-WT or PARP2-QFRD (ethidium bromide staining). B: Cryo-electron micrograph of the PARP2-QFRD–Nuc165 complex. Enlarged views of two particles are shown (1 and 2). Scale bar: 50 nm. Cartoon representations of the two particles is presented alongside the enlarged views. C: Domain structure of human PARP2 and location of the Q112R and F113D double point mutation in the WGR domain. D: Location of the Q112 and F113 residues (drawn as red sticks) in the context of the PARP2-WGR–DNA complex crystal structure (PDB entry 6F5B).