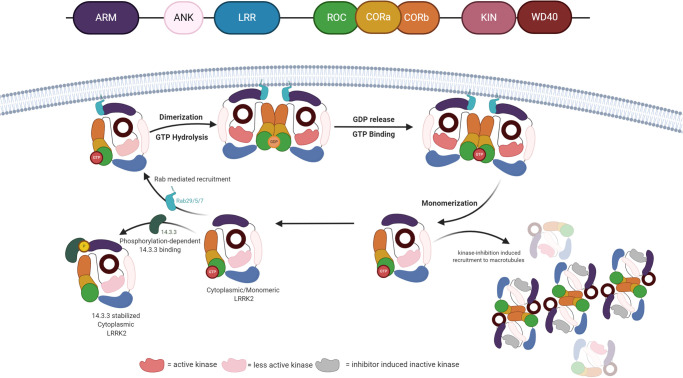
Figure 1. LRRK2 oscillate between different conformation for full kinase activation.
LRRK2 is mainly present in a monomeric GTP-bound form in the cytosol. This state is less active and can form a stable cytoplasmic complex with 14-3-3 proteins. Binding of activated Rab proteins induces membrane localization of LRRK2. At the membrane, GTP is hydrolyzed and the protein dimerizes. During the GTPase cycle, the LRRK2 kinase domain gets fully activated and phosphorylates its substrates. The low affinity of LRRK2 for GDP facilitates fast GDP release, rebinding of GTP and, subsequently monomerization of LRRK2 and return to the cytosol. Conventional pharmacological inhibition of LRRK2 induces microtubule recruitment and formation of filamentous LRRK2, dimeric units of LRRK2 extended via WD40 : WD40 or LRR : LRR interaction.