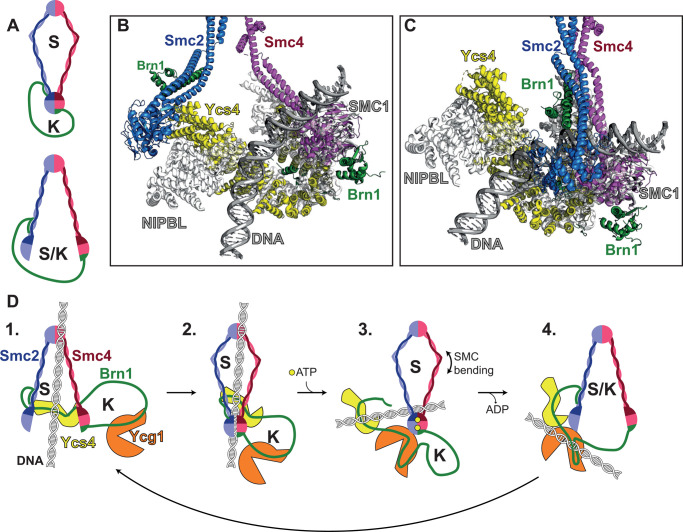
Figure 3. A potential mechanism for condensin DNA binding.
(A) SMC complexes can create two distinct DNA entrapment compartments, the S-compartment (S), made by the SMCs and the K-compartment (K), made by the Kleisin. Opening of the SMC ATPase heads could result in fusion of these compartments (S/K). (B) Overlay of the S. cerevisiae bridged Apo condensin structure (coloured by domain, PDB: 6YVV) with NIPBL, SMC1 ATPase domain and DNA from the H. sapiens cohesin structure (shown in white/grey, PDB: 6WG3). This places DNA in contact with the top of the Smc4 ATPase domain and a region of Ycs4 with positive surface charge, discussed by Lee and Merkel et al. [59]. (C) Overlay of the S. cerevisiae Apo condensin structure (PDB: 6YVU) with NIPBL, SMC1 ATPase domain and DNA from the H. sapiens cohesin structure (PDB: 6WG3), suggesting the ATPases heads clamp around DNA after it is bound by Ycs4. (D) The proposed mechanism for condensin DNA binding during ATP turn over.