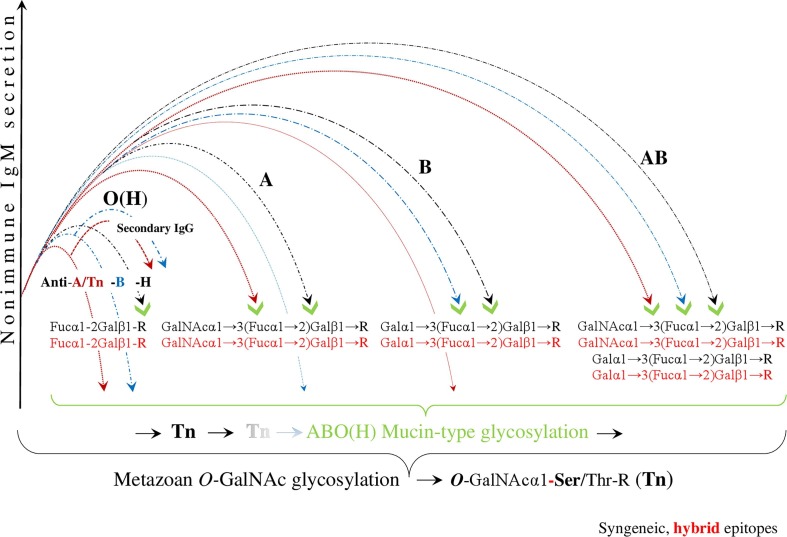
Fig. 2.
Evading immunity by ABO(H) blood group hybridization. After SARS-CoV-2 infection, initiated by trans-species, metazoan O-GalNAc-glycosylation, the virus hypothetically invades human cells via hybridization of the ABO(H) blood groups. This results in the downregulation and/or reduction of the innate anti-ABO(H) isoagglutinin reactivity, exerted by polyreactive nonimmune IgM. In this figure, the virtual, hybrid mucin-type epitopes mimic syngeneic epitopes, which are the targets of this hybridization. The blood group O(H), which is bound to the virus solely by the formation of the hybrid H-type antigen, has the least amount of contact with the pathogen and is the most protected group when it loses only the anti-H, but retains innate anti-A and anti-B isoagglutinin reactivity, involving secondary IgG responses. In blood group A, the anti-A and anti-H formations are physiologically blocked, a decrease in the level of anti-B isoagglutinin is observed, and neither anti-A- nor anti-B-reactive IgG is produced. In blood group B, the anti-B and anti H-reactivities are blocked, a decrease in the level of anti A is observed, and, similar to blood group A, neither anti-A nor anti-B-reactive IgG is produced. Blood group AB has the strongest contact to the pathogen and is the least protected group with respect to innate ABO(H) immunity. (Because the first contact between the SARS-CoV-2 virus and the host organism, and the first contact between the protozoan parasite P-falciparum and the host organism are hypothetically identical, similar images were used for the illustrations (Arend, 2020b).