Fig. 2.
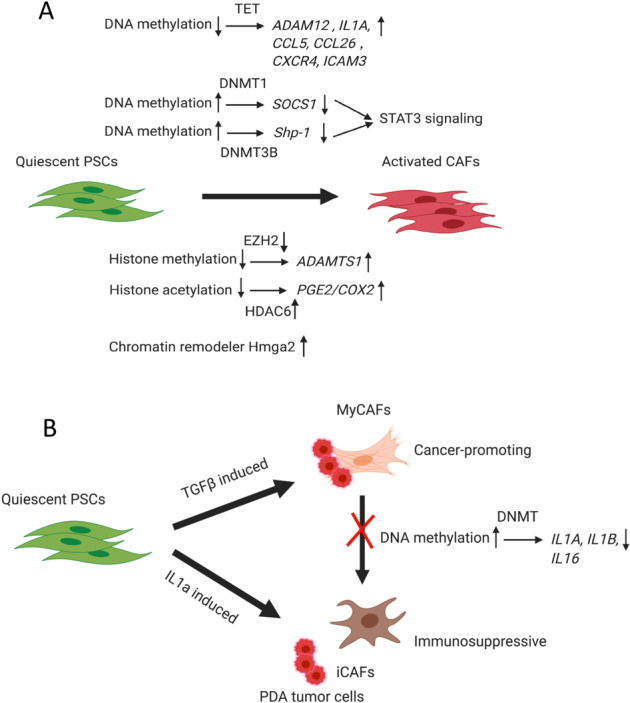
Epigenetic regulation in cancer-associated fibroblasts. a DNA methylation of SOCS1 regulated by DNMT1 and Shp-1 regulated by DNMT3B leads to gene repression, which activates STAT3 signaling during CAF differentiation and activation. DNA hypomethylation by TET, which leads to the upregulation of ADAM12, IL1A, CCL5, CCL26, CXCR4, and ICAM3 and triggers CAF differentiation. Decreased histone methylation regulated by EZH2 results in the upregulation of ADAMTS1 during CAF activation. Increased histone acetylation regulated by HDAC6 controls the upregulation of PGE2/COX2 during CAF activation. The chromatin remodeler Hmga2 is also induced to facilitate CAF activation. b Quiescent PSCs can be differentiated into either myCAFs or iCAFs, while myCAFs and iCAFs can also be reprogrammed interchangeably. MyCAFs can be induced by TGFβ signaling through direct contact with PDA tumor cells, while iCAFs can be induced by paracrine IL1a signaling through indirect interaction with tumor cells. MyCAFs have a cancer-promoting phenotype, while iCAFs have an immunosuppressive phenotype. DNA methylation of the IL1A and IL1B genes and their subsequent downregulation were observed in human MSCs cocultured with PDA tumor cells because of direct interaction, which potentially locked CAFs into the myCAF phenotype and prevented the transformation of myCAFs into iCAFs, supporting tumor growth
