Fig. 5.
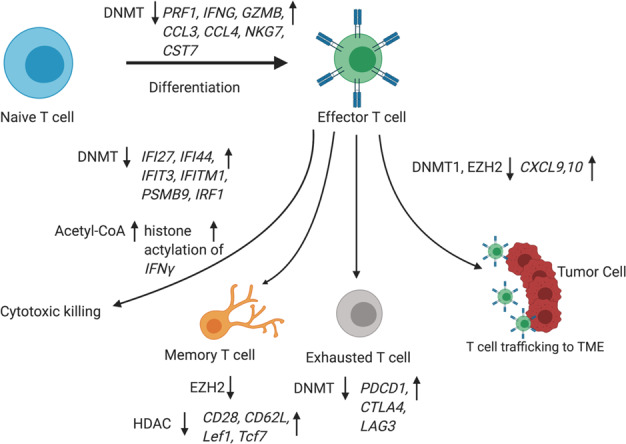
Epigenetic regulation of T lymphocytes. DNMT downregulation promotes Th1 polarization and activation of cytotoxic T cells because of the upregulation of IFNγ-stimulated genes, such as IFI27, IFI44, IFIT3, IFITM1, PSMB9, and IRF1. EZH2-mediated histone H3 lysine 27 trimethylation (H3K27me3) and DNA methyltransferase 1 (DNMT1)-mediated DNA methylation repress the expression of chemokines CXCL9 and CXCL10 and subsequently prevent effector T-cell trafficking to the TME. DNMT downregulation leads to the upregulation of signature genes in cytotoxic T cells, including PRF1, IFNG, GZMB, CCL3, CCL4, NKG7, and CST7, during naive T-cell to effector T-cell differentiation. Three inhibitory receptors, PDCD1, CTLA4, and LAG3, were found to be specifically demethylated within tumor-reactive CD8+ T cells and in late stages of T-cell subtype activation. HMT EZH2 negatively regulates the differentiation of memory T cells from effector T cells. Induction of acetyl-coenzyme A during aerobic glycolysis enhances the histone acetylation and transcriptional activation of Ifng in activated T cells. Histone deacetylase inhibition was shown to reprogram differentiated human CD8+ T cells into central memory-like T cells synergistically with IL21 and the upregulation of central memory T-cell surface CD28 and CD62L, showing a stable memory-associated transcriptional signature with increased Lef1 and Tcf7
