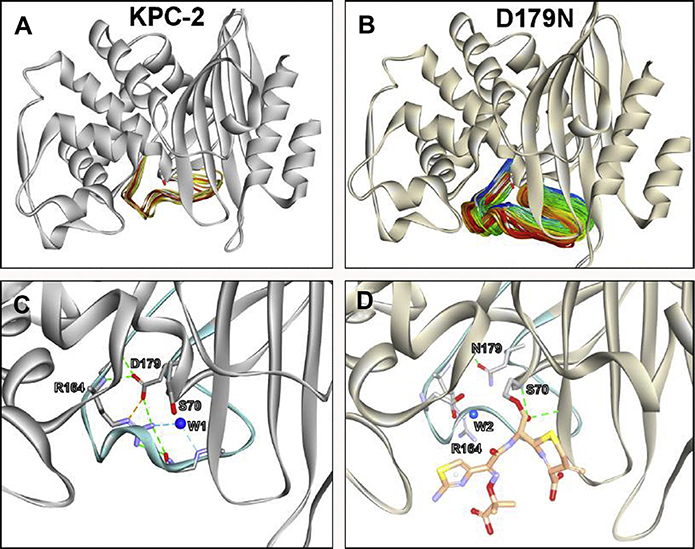
Fig. 8.
Molecular modeling and 500-ns molecular dynamic simulation revealed the flexibility and mobility of the Ω-loop was increased in the (B) KPC-2 D179N variant due to disruption of the salt bridge with R164; mobility is RMSD of (A) 2 Å in KPC-2 versus (B) 10 Å for D179N variant. (C) In KPC-2, the R164 residue forms a salt bridge with D179 and hydrogen bonding network with a water molecule (W1). (D) The substitution D179N disrupts the salt bridge, and the nucleophilic S70 and the general base, E166 are repositioned, which results in the repositioning of the catalytic water (W2) and the formation of a longer-lasting acyl-enzyme complex with the variant and ceftazidime.