Abstract
Although previous reports described the beneficial role of angiotensin-converting enzyme inhibitors (ACE-Is) or AT1 receptor blockers (ARBs) in attenuating neuropathic pain (NP), no study has yet explored the exact underlying mechanisms, as well as the superiority of using centrally versus peripherally acting renin–angiotensin–aldosterone system (RAAS) drugs in NP. We investigated the effects of 14 days of treatment with centrally (telmisartan and ramipril) or peripherally (losartan and enalapril) acting ARBs and ACE-Is, respectively, in attenuating peripheral NP induced by sciatic nerve chronic constriction injury (CCI) in rats. We also compared these with the effects of pregabalin, the standard treatment for NP. Behavioral changes, inflammatory markers (NFкB, TNF-α, COX-2, PGE2, and bradykinin), oxidative stress markers (NADPH oxidase and catalase), STAT3 activation, levels of phosphorylated P38-MAPK, ACE, AT1 receptor (AT1R), and AT2 receptor (AT2R), as well as histopathological features, were assessed in the brainstem and sciatic nerve. CCI resulted in clear pain-related behavior along with increased levels of inflammatory and oxidative stress markers, and STAT3 activity, as well as increased levels of phosphorylated P38-MAPK, ACE, AT1R, and AT2R, along with worsened histopathological findings in both the brainstem and sciatic nerve. ARBs improved both animal behavior and all measured parameters in CCI rats and were more effective than ACE-Is. At the tested doses, centrally acting ARBs or ACE-Is were not superior to the peripherally acting drugs of the same category. These findings suggest that ARBs (centrally or peripherally acting) are an effective treatment modality for NP.
Electronic supplementary material
The online version of this article (10.1007/s13311-020-00912-8) contains supplementary material, which is available to authorized users.
Keywords: Neuropathic pain, P38 MAPK, STAT3, ACE-Is, ARBs
Introduction
Peripheral nerve damage causes abnormal chronic pain and pain hypersensitivity, which is referred to as “neuropathic pain” (NP) [1]. The damage can involve peripheral fibers (Aβ, Aδ, and C fibers) or central neurons and results in different pain sensations, including hyperalgesia, allodynia, spontaneous pain, and chronic inflammation [2]. Ongoing injury overstimulates the release of inflammatory mediators, such as TNF-α and NF-KB, from nociceptive terminals along with increased vascular permeability and the accumulation of prostaglandins, bradykinin, different cytokines, and reactive oxygen species [3]. The complexity and involvement of several mediators in NP may partly explain why no drug is universally effective for this condition.
The lack of effective treatments for NP and various unclear mechanisms behind it justify the need to explore novel drugs [4] or utilize the analgesic properties of drugs currently used to treat conditions not related to pain. Among these drugs, angiotensin-converting enzyme inhibitors (ACE-Is) and angiotensin receptor blockers (ARBs) are drawing particular attention [5]. Apart from its well-documented cardiovascular effects [6], stimulation or inhibition of the renin–angiotensin–aldosterone system (RAAS) can modulate pain perception via different mechanisms, which are still poorly understood [7].
Drugs blocking activation of the RAAS, such as renin inhibitors, ACE-Is, ARBs, and aldosterone antagonists, showed clinical significance in migraine, neuropathic, and nociceptive pain in different animal models [8, 9]. Their favorable outcomes were attributed to the inhibition of an inflammatory cascade by inhibiting the production of key cytokines, including tumor necrosis factor (TNF)-α [10–12].
Angiotensin signaling can activate a number of mediators and stressors that are involved in the pathogenesis of NP. These include P38 mitogen activated protein kinase (p38 MAPK) [13, 14], the Janus kinase 2/signal activator and transducer of transcription 3 (JAK2/STAT3) pathway [15, 16], nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2) [17, 18], and inducible nitric oxide synthase (iNOS). Moreover, different proinflammatory cytokines such as interleukin-1β (IL-1β), TNF-α, and interleukin-6 (IL-6) can also be activated by the same system [19–22]. Despite the ability of angiotensin II (Ang II) (via AT1Rs) to activate MAPKs and the JAK2/STAT3 pathway in the brainstem and cerebellum primary astrocytes [15, 23], as well as this pathway’s critical role in mediating NP following peripheral nerve injury at the spinal level [16, 24], the possible protective role of RAAS modulators at both peripheral and supraspinal levels via this pathway remains elusive. Moreover, to the best of our knowledge, no study has yet investigated whether a centrally acting (crosses the BBB) RAAS drug is preferable to a peripherally acting (does not or poorly crosses the BBB) one in mitigating NP. Our study aimed to investigate the ability of RAAS blockers to mitigate NP via modulating p38MAPK-JAK-STAT3 signaling. In addition, the study investigated angiotensin receptor blockade versus ACE inhibition, as well as central versus peripheral RAAS targeting to alleviate NP, utilizing both peripherally and centrally acting ACE-Is and ARBs in a well-established model of NP, the chronic constriction injury (CCI) model [25, 26].
Materials and Methods
Drugs and Chemicals
Telmisartan, losartan potassium, enalapril maleate, ramipril, and pregabalin were purchased from Sigma-Aldrich (St. Louis, MO) and were suspended in 0.5% carboxymethyl cellulose using distilled water. Thiopental, penicillin G, and acetone were purchased from Eipico Pharmaceuticals (10th of Ramadan, Egypt).
Experimental Animals
Male albino rats, weighing 250 to 280 g were purchased from the Faculty of Veterinary Medicine, Zagazig University, Egypt. They were housed in pairs with free access to water and standard feed. The animals were exposed to a normal cycle of light and dark under a 12/12-h reversed light–dark cycle (dark: 08:00 to 20:00 h) and maintained under controlled temperature and humidity (23 °C ± 2 °C and 60% ± 10%, respectively). Experiments on the rats were started after an acclimatization period of 1 week and were carried out in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (NIH) and with The Code of Ethics of the World Medical Association. All animal protocols were approved by the Institutional Animal Care and Use Committee at Zagazig University (ZU-IACUC), Egypt.
Induction of Neuropathic Pain by Chronic Constriction Injury
Unilateral CCI of the sciatic nerve was induced as previously reported [27]. The rats were anesthetized with thiopental sodium (50 mg/kg, i.p.). After depilation of the right hind limb, the sciatic nerve was exposed under aseptic conditions at the midthigh level beneath the gluteus and biceps femoris muscles. A 7-mm-long common sciatic nerve segment proximal to the trifurcation was separated from the surrounding tissue. The procedure was ended for group 2 (sham) at this point. In the remaining groups, CCI was induced by placing 4 loose ligatures (4/0 silk suture) around the nerve at intervals of 1 mm. Then, the wound was closed. The animals were returned to their cages to recover. A single dose of penicillin G was given just prior to surgery according to the used method.
Behavioral Examination
The behavioral tests measured foot withdrawal thresholds in response to stimuli applied to the injured hind paw. For each test, the animal was placed in a plastic chamber (15 × 15 × 25 cm) and habituated for at least 15 min to become accustomed to the laboratory environment. The chamber was placed on top of a mesh screen to enable mechanical stimuli administration to the plantar surface of the injured hind paw.
Paw Cold Allodynia (Acetone Drop Test)
Briefly, as previously described by Flatters and Bennett [28], 100 μL of acetone was sprayed onto the surface of the injured paw of a rat (placed over a wire mesh), without touching the skin. The response of the rat was noted for 20 s and was graded to a 4-point scale, in which 0 means no response; 1, quick withdrawal or flick of the paw; 2, prolonged withdrawal or repeated flicking; and 3, repeated flicking with licking of the paw. Acetone was applied 3 times to the injured hind paw, with a gap of 5 min between the acetone applications. The individual scores noted in a 20-s interval were added to obtain a single score over a cumulative period of 60 s. The minimum score was 0, whereas the maximum possible score was 9.
Heat Hyperalgesia (Hot Plate Test)
The thermal nociceptive threshold, as an index of thermal hyperalgesia, was assessed using a hot plate, maintained at a temperature of 52.5 °C ± 1.0 °C. The rat was placed on the hot plate, enclosed in a plexiglass cage, and the withdrawal latency to heat-induced nociceptive pain was recorded. The cut-off time of 20 s was maintained to avoid potential tissue damage [29, 30].
Mechanical Hyperalgesia (Pinprick Test)
As described by Erichsen and Blackburn-Munro [31], the mid–plantar surface of the injured hind paw was touched with the point of the bent gauge needle (at 90° to the syringe) with an intensity sufficient to produce a reflex withdrawal response without penetrating the skin. The duration of paw withdrawal in seconds was recorded. A cut-off time of 20 s was maintained to avoid wind-up phenomena. The duration of withdrawal response was recorded with an arbitrary minimum value of 0.5 s (for the brief normal response) and a maximum value of 20 s.
Mechanical Dynamic Allodynia (Paintbrush Test)
To explore dynamic responses to a mechanical stimulus, we used a smooth paintbrush to produce allodynia as normal rats never withdraw from this stimulus. In brief, each rat was placed on an elevated wire mesh floor covered with a glass cage, after which a smooth paintbrush was used to rub the plantar area of the hind paw from the heel to the toes as a stimulus. The stimulus was applied 5 times with 5-s intervals, and the number of withdrawals was noted (between 0 and 5). The procedure was applied 3 times for each rat, with intervals of 5 min, and the total number of withdrawals (in 3 tests) was determined to obtain a single cumulative score of mechanical dynamic allodynia, having a minimum value of 0 and maximum of 15 [32, 33].
Experimental Design
The rats (n = 64) were randomly and equally divided into 8 groups, as follows: group I, normal: the rats received the vehicle [0.5% carboxymethylcellulose solution (10 ml/kg, p.o.)] for 14 days; group II (sham): the rats were subjected to a surgical procedure to expose the right sciatic nerve on day 1 without any nerve ligation and received the vehicle for 14 days; group III (control): the rats were subjected to CCI and received the vehicle; groups IV to VIII: the rats were subjected to CCI and received the peripherally acting ACE inhibitor enalapril [34] (10 mg/kg, p.o.), the peripherally acting ARB losartan [35] (20 mg/kg, p.o.), the centrally acting ACE inhibitor ramipril [36] (4 mg/kg, p.o.), the centrally acting ARB telmisartan [37] (5 mg/kg, p.o.), or the standard treatment of NP, pregabalin (10 mg/kg, p.o.), respectively for 14 days. Another group of sham rats were divided to receive the individual treatments similar to the CCI groups. We selected the doses in this study based on the relative potencies of ARBs and ACE-Is. At the same doses, telmisartan is nearly 4 times more effective compared with losartan [38], whereas ramipril has a relatively higher potency [39] and longer half-life [40], and is prescribed in half doses compared with that of enalapril. Further, the doses are similar to those used in other studies for enalapril [41], losartan [42, 43], ramipril [44], telmisartan [9, 45], and pregabalin [44]. All drugs were given by oral gavage once daily at the same time in the morning. The animals were tested for behavioral responsiveness by a blinded experimenter 1 h following drug administration on day 1 before surgery and then on days 7 and 14 prior to sacrifice [46]. A preliminary study (n = 3/group) was conducted to verify the experimental rationale. The sample size was estimated using GraphPad StatMate 2 statistical program (GraphPad Software, Inc., La Jolla, CA).
Biochemical Analysis in Sciatic Nerve and Brainstem Tissue
At the end of the study period, and following the last behavioral assessment, blood samples were collected via cardiac puncture under anesthesia with ketamine/xylaxine (90 mg/kg/9 mg/kg, i.p.) followed by removal of the heart to ensure euthanasia. Determination of the peripheral and central contributions to the progression of NP requires investigation of the anti-inflammatory potential of RAAS-modulating drugs in the sciatic nerve and in the brainstem, which is known to be greatly involved in the maintenance of NP [2, 47]. Following blood collection, ipsilateral sciatic nerves (~ 10 mm), including the injured site and approximately 5 mm of surrounding tissues on both sides, were excised. Brainstems were then harvested. Tissues were either preserved in liquid nitrogen or kept in formalin for the histochemical analysis. Tissues were homogenized in cold 50 mM phosphate-buffered saline (pH 7.4) by a glass homogenizer (Omni International, Kennesaw, GA). The homogenate was centrifuged at 3000 rpm for 10 min at 4 °C. The protein content of the supernatant was determined using the Bradford assay (Bio-Rad, Hercules, CA). ELISA kits were used to measure the levels of COX-2, ACE, NADPH oxidase, catalase (Cusabio, Houston, TX), AT1R, AT2R, iNOS, NF-KB, bradykinin, PGE2 (MyBioSource, San Diego, CA), and TNF-α (Sigma-Aldrich), as well as P38 MAPK total and phosphorylated levels (Invitrogen, Carlsbad, CA), and STAT-3 phosphorylated and total levels (RayBiotech, Mountain View, CA), as per the manufacturers’ instructions.
Histopathological Examination
Sciatic nerve and brainstem tissue samples were preserved in 10% buffered formalin and processed for routine paraffin block preparation. Using an American optical rotary microtome, sections around 5 μm in thickness were cut and stained with hematoxylin and eosin (H&E). The sections were evaluated using a bright-field microscope and photographed. Quantification of myelin damage of the sciatic nerves of the different groups was done using the Nerve Injury Scoring System (NISS), using the following scores: 1 = normal, mild degeneration, or demyelination; 2 = moderate level of degeneration (< 50% damaged nerve tissue); and 3 = diffuse degeneration or demyelination (> 50% damaged nerve tissue) [48].
Immunohistochemical Staining of Myelin Basic Protein and Glial Fibrillary Acidic Protein
Transverse sciatic nerve or brainstem paraffin sections (5 μm in thickness) were deparaffinized, then rehydrated through a series of graded ethanol concentrations. Endogenous peroxides were inactivated by incubating with 3% H2O2 in PBS for 10 min. Sciatic nerve and brainstem sections were processed for myelin basic protein (MPB) and the astrocyte marker glial fibrillary acidic protein (GFAP), respectively. Immunohistochemical staining was performed using MPB (1:100) or GFAP (1:200) primary antibodies (Millipore Sigma, Burlington, MA) for 24 h at 4 °C. Sections were then incubated with biotinylated secondary antibody and avidin–biotin complex (Vectastain® ABC-peroxidase kit, Vector Laboratories, Burlingame, CA). Color was developed with 3,3′-diaminobenzidine (DAB) substrate (Vector® DAB, Vector Laboratories). The Image J analysis software (Fiji Image J; 1.51 n, NIH, USA) was used to estimate the area percentage of myelin basic protein (MBP) or GFAP reactivity at × 400 magnification.
Statistical Analysis
Data are expressed as mean ± SEM. Statistical analysis were carried out using 1-way ANOVA followed by Bonferroni’s post hoc test, Kruskal–Wallis tests (nerve injury scoring), or repeated-measures ANOVA followed by Bonferroni’s post hoc test (behavioral tests). p values ≤ 0.05 were considered statistically significant. All statistical tests were conducted using GraphPad Prism (version 5) software (GraphPad Software, Inc.).
Results
Behavioral Results
There were no significant differences between the normal and sham groups. Therefore, all comparisons were performed with respect to the sham group. Notably, per se administration of different treatments did not significantly modulate the assessed behavioral responses (data not shown).
Effect of Different Interventions on Cold Allodynia and Heat Hyperalgesia in CCI Model of Neuropathy
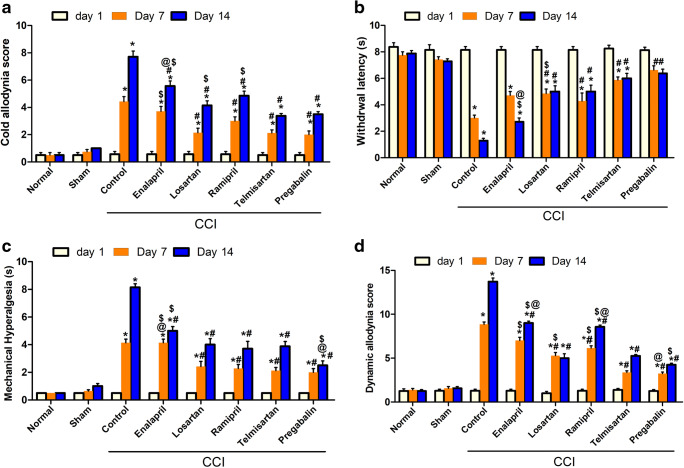
Rats exposed to CCI, when assessed on day 14, showed increased cold allodynia (Fig. 1A) and heat hyperalgesia responses (Fig. 1B), represented as a significant (p < 0.001) decrease and increase in heat response latency time and cold allodynia score by 6- and 14-fold, respectively, compared with the levels in the sham group. On the other hand, rats treated with RAAS-modulating drugs showed significantly lower heat hyperalgesia and cold allodynia responses when measured on day 14 post-CCI. Notably, ARBs (telmisartan and losartan) showed superiority over ACE-Is in the cold allodynia test, and their effects were comparable to those obtained in rats treated with the standard drug for NP, pregabalin. Meanwhile, in the hot plate test, which is mostly used to test central analgesic activity, centrally acting agents (telmisartan and ramipril) showed superiority over peripherally acting ones (enalapril and losartan) (Fig. 1A).
Fig. 1.
Effect of different interventions on behavioral changes: (A) cold allodynia assessed by acetone drop test, (B) heat hyperalgesia assessed by hot plate test, (C) mechanical hyperalgesia assessed by pinprick test, and (D) mechanical dynamic allodynia assessed by paintbrush test before (day 1) and on the 7th and 14th days post-CCI. Data is expressed as mean ± SEM (n = 7-8) per group. *p < 0.05 versus sham group; #p < 0.05 versus CCI group; $p < 0.05 versus telmisartan group; @p < 0.05 versus losartan group, at the corresponding time points. CCI = chronic constriction injury
Effect of Interventions on Mechanical Hyperalgesia
CCI resulted in a significant (p < 0.001) increase in mechanical hyperalgesia as assessed by the pinprick test on days 7 and 14, as indicated by an increase in the withdrawal time of injured hind paw compared with that in the sham group (4.1 ± 0.3 vs 0.5 ± 0.0 and 8.1 ± 0.3 vs 0.5 ± 0.0 s, respectively) (Fig. 1C). This increase was similarly attenuated by all tested compounds, starting at day 7 post-CCI, with the exception of enalapril, which showed the weakest effect.
Effect of Various Drugs on Mechanical Dynamic Allodynia
As shown in Fig. 1D, CCI resulted in a significant (p < 0.001) increase in dynamic allodynia score as assessed by the paintbrush test, when measured at the 7th and 14th days postsurgery, compared with the levels in the sham group (8.9 ± 0.3 vs 1.6 ± 0.2 and 13.7 ± 0.4 vs 1.3 ± 0.2 withdrawals, respectively). All tested drugs attenuated dynamic allodynia with ARBs showing superiority over ACE-Is. In this experiment, no significant difference was observed between central and peripheral interventions within the same class. Notably, losartan-treated rats showed findings comparable to those of pregabalin-treated ones (5.0 ± 0.5 and 4.3 ± 0.2 paw withdrawals, respectively).
Biochemical Parameters
Effect of Different Treatment Modalities on Oxidative Status
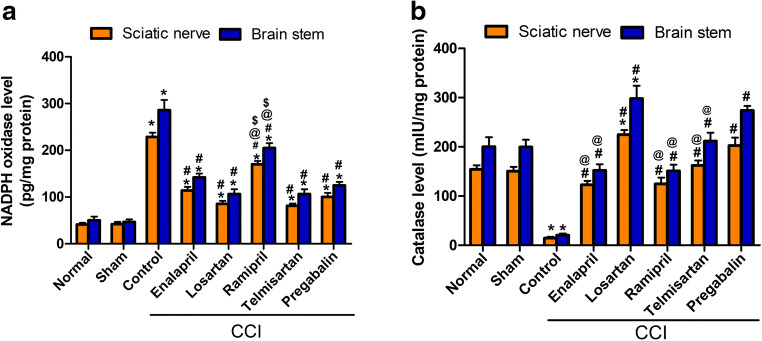
CCI rats exhibited significant (p < 0.001) increases in NOX level (5.4- and 6.2-fold) (Fig. 2A), along with significant (p < 0.001) reductions (90.3% and 89.7%) in catalase activity (Fig. 2B), in both sciatic nerve and brainstem tissues, respectively. Rats treated with different drugs acting on RAAS showed lower NOX levels, with the ARB-treated groups showing superior effects over ACE-I-treated groups and effects equal to those of pregabalin. Ramipril showed mild effects (25% and 28% reductions) in sciatic and brainstem tissues, respectively. Moreover, CCI rats treated with drugs acting on RAAS showed elevated levels of catalase. Interestingly, the losartan-treated group showed a higher sciatic nerve and brainstem catalase level compared with those of the sham group (224.8 ± 9.5 mlU/ml vs 150.6 ± 8.7 mlU/ml) and (298.8 ± 26.1 mlU/ml vs 199.6 ± 15.0 mlU/ml), respectively.
Fig. 2.
Effect of different treatment modalities on oxidative status in sciatic nerve and brainstem: (A) effect on NADPH oxidase level and (B) effect on catalase level. Data is expressed as mean ± SEM (n = 5-6) per group. *p < 0.05 versus sham group; #p < 0.05 versus CCI group; @p < 0.05 versus losartan group; $p < 0.05 versus telmisartan group at the corresponding time points. CCI = chronic constriction injury
Effect of Different Treatment Modalities on NF-Kβ and TNF-α
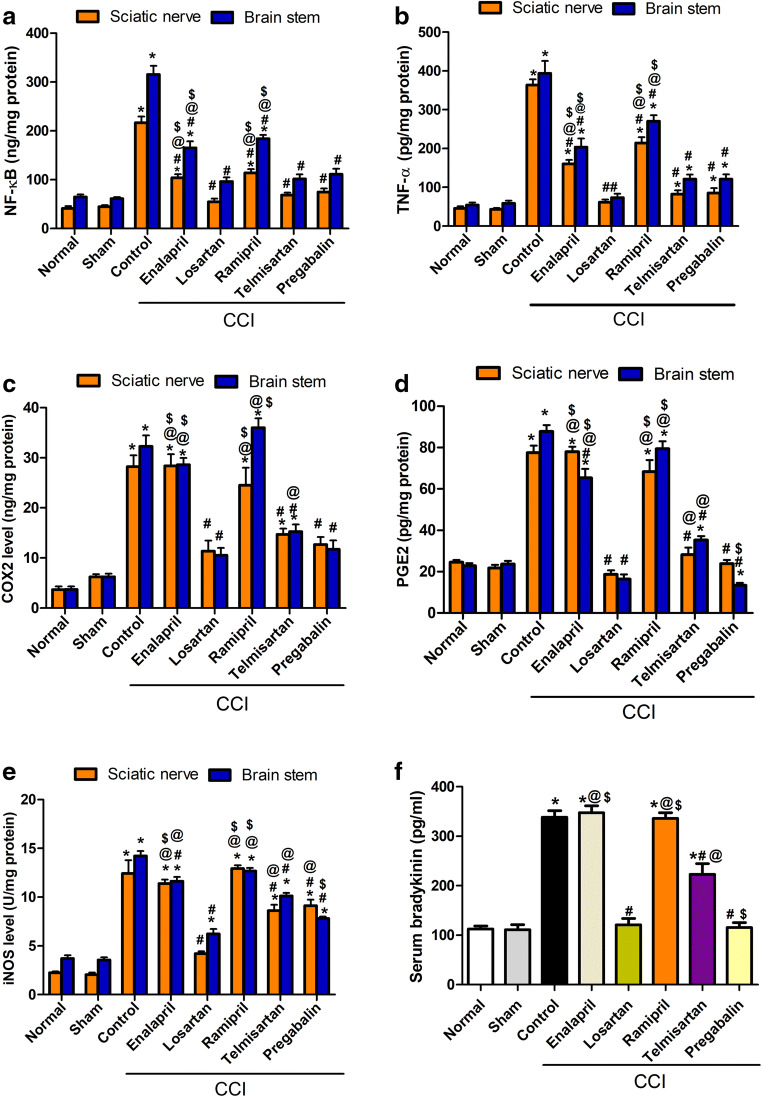
Chronic constriction of the sciatic nerve resulted in significant increases (p < 0.001) in the levels of NF-Kβ (4.1- and 3.9-fold) and TNF-α (5.6- and 7.6-fold), in brainstem and sciatic tissues, respectively, compared with the sham group (Fig. 3A, B). The previous effects were attenuated by all tested compounds to different degrees, with ARBs (telmisartan and losartan) being the most effective as they restored both parameters to near-normal levels. Moreover, the effects of ARBs were comparable to that of pregabalin, the standard drug used for treating NP.
Fig. 3.
Effect of various treatment modalities on CCI-induced increased levels of (A) NF-кβ, (B) TNF-α, (C) COX-2, (D) PGE2, (E) iNOS in sciatic and brainstem tissues, and (F) bradykinin in serum. Data is expressed as mean ± SEM (n = 5-6) per group. *p < 0.05 versus sham group; #p < 0.05 versus CCI group; @p < 0.05 versus losartan group; $p < 0.05 versus telmisartan group at the corresponding time points. CCI = chronic constriction injury; NF-кβ = nuclear factor kappa β; TNF-α = tumor necrosis factor-α; COX2 = cyclooxygenase 2; PGE2 = prostaglandin E2; iNOS = inducible nitric oxide synthase
Effect of Different Treatment Modalities on COX-2, PGE2, iNOS, and Bradykinin
CCI significantly (p < 0.001) increased the levels of COX-2, PGE2, and iNOS in both brainstem (4.0-, 2.7-, and 3.0-fold) and sciatic nerve tissues (3.5-, 2.5-, and 5.2-fold), respectively, compared with those in the sham group. The previous effects were significantly (p < 0.05) reduced in the ARB-treated groups only in both tissues (Fig. 3C–E). Moreover, the serum level of bradykinin was elevated (p < 0.001) by CCI (threefold) compared with that in the sham group (Fig. 3F). Meanwhile, the ARB-treated groups showed a reduction on its level; ACE-Is did not show any significant reduction compared with the level in the control CCI group. Losartan restored the levels of bradykinin to normal, similar to pregabalin. Notably, losartan was the most effective among the tested drugs in ameliorating the levels of COX2, PGE2, and iNOS in serum, sciatic nerve, and brainstem.
Effect of Various Drugs on STAT-3 and P38 MAPK Phosphorylation
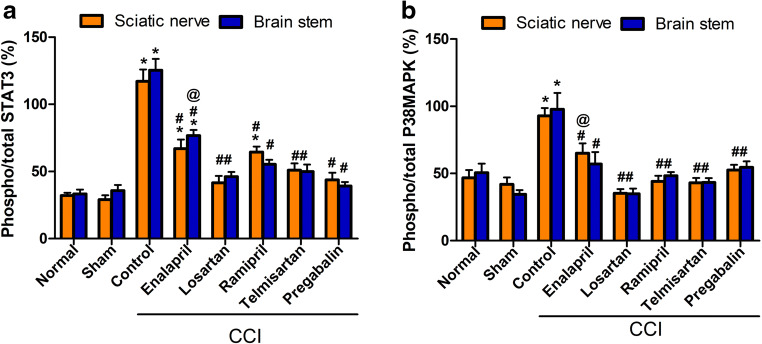
CCI resulted in a significant (p < 0.001) increase in the phosphorylated/total STAT-3 ratio in both brainstem and sciatic tissues by 2.5- and 3-fold, respectively, compared with that in the sham group. Meanwhile, all tested drugs attenuated the latter response to different degrees. Losartan-, telmisartan-, and pregabalin-treated groups showed a phosphorylated/total STAT-3 ratio (p < 0.05, Fig. 4A) that was not significantly different from that in the sham group. Moreover, CCI resulted in a significant increase (p < 0.05, Fig. 4B) in phosphorylated/total P38 MAPK ratio in both brainstem and sciatic tissues, by 1.8- and 1.2-fold, respectively, compared with that in the sham group. All treatment modalities attenuated the previous effect with no significant difference among all of the groups (p < 0.05, Fig. 4B).
Fig. 4.
Effect of different treatment modalities on (A) phospho/total STAT3 percentage and (B) phospho/total P38-MAPK percentage in both sciatic nerve and brainstem tissues. Data is expressed as mean ± SEM (n = 5-6) per group. *p < 0.05 versus sham group; #p < 0.05 versus CCI group; @p < 0.05 versus losartan group at the corresponding time points. CCI = chronic constriction injury; phospho-STAT3 = phosphorylated signal transducer and activator of transcription 3; P38-MAPK = p38, mitogen activated protein kinase
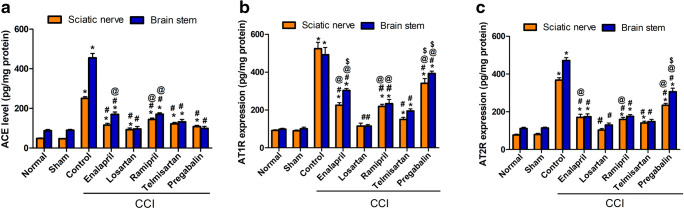
Effect of Different Treatment Modalities on ACE Level, AT1 R, and AT2R Expression
The rats subjected to CCI exhibited significant (p < 0.0001) increases in ACE level and AT1 R expression (harmful arm of the RAAS axis) in brainstem (fivefold) and sciatic tissues (5.3- and 5.8-fold), respectively, compared to those in the sham group (Fig. 5A, B). Losartan (20 mg/kg, p.o.) was the only drug able to restore normal values for both preparations, an effect that was superior to that of pregabalin on ACE levels while comparable to it on AT1R level. The other treatment modalities reduced the levels of ACE compared with that in the CCI group (p < 0.05) to varying degrees, with telmisartan being superior over ACE-Is. Notably, telmisartan showed 60% and 75% reductions in ATIR levels compared with those in the sham group in sciatic and brainstem tissues, respectively (p < 0.05), which was more effective than ACE-Is (Fig. 5B). Moreover, the expression of AT2R in the brainstem and sciatic nerve was significantly higher in CCI rats (by fourfold) in both tissues (p < 0.05, Fig. 5C). All the RAAS-acting drugs decreased the expression level of AT2R to varying degrees. Both losartan and telmisartan succeeded in restoring AT2R levels to near normal in the brainstem, whereas in sciatic tissues, losartan was superior to all other treatments (p < 0.05, Fig. 5C).
Fig. 5.
Effect of different treatment modalities on (A) ACE level, (B) AT1R expression, and (C) AT2R expression, in both sciatic and brainstem tissues. Data is expressed as mean ± SEM (n = 5-6) per group. *p < 0.05 versus sham group; #p < 0.05 versus CCI group; @p < 0.05 versus losartan group; $p < 0.05 versus telmisartan group at the corresponding time points. CCI = chronic constriction injury; ACE = angiotensin converting enzyme; AT1R = angiotensin type 1 receptor; AT2R = angiotensin type 2 receptor
Brainstem and Sciatic Nerve Histopathological Examination
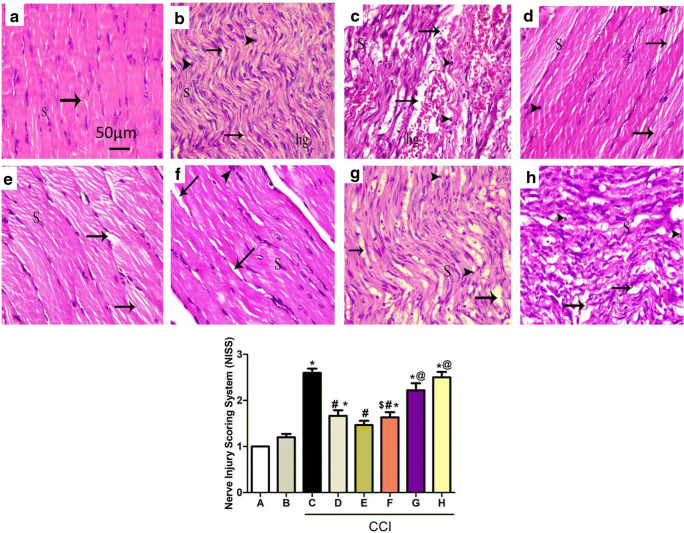
Effect on Sciatic Nerves
Sections of the sciatic nerve from the normal group stained by H&E showed well-defined myelin sheaths and the absence of infiltrating cells, with a normal number of Schwann cell nuclei (Fig. 6A). Sciatic sections of the sham group also showed well-organized wavy myelin sheaths with infiltrating mononuclear cells and slight hemorrhage between the myelin sheaths (Fig. 6B). Meanwhile, stained nerve sections obtained from CCI rats showed several areas of edema and degraded wavy myelin sheaths with hemorrhage in between, with mononuclear infiltrating cells and Schwann cell nuclei (Fig. 6C). Additionally, sections obtained from the group treated with pregabalin, the standard drug for NP, were used for comparison of the histopathological changes (Fig. 6D).
Fig. 6.
Upper panels: photomicrograph (H&E, × 400) of longitudinal sciatic nerve sections from normal (A), sham (B), CCI (C), pregabalin (D), losartan (E), enalapril (F), telmisartan (G), and ramipril (H). Rats received different treatments for 14 days, then they were sacrificed and tissues were collected. Arrows and arrowheads illustrate myelin sheaths’ degeneration and mononuclear infiltrating cells, respectively. S = Schwan cell nuclei; hg = hemorrhage. Scale bar, 50 μm. Lower panel: quantification of myelin damage of sciatic nerves of different groups represented in A–H using Nerve Injury Scoring System (NISS), and the scoring was 1 = normal, mild degeneration, or demyelination; 2 = moderate level of degeneration (< 50% damaged nerve tissue); and 3 = diffuse degeneration or demyelination (> 50% damaged nerve tissue). *p < 0.05 versus sham group; #p < 0.05 versus CCI group; @p < 0.05 versus losartan group; $p < 0.05 versus telmisartan group
Sections obtained from CCI rats treated with losartan, enalapril, telmisartan, and ramipril showed improvements to varying degrees. Notably, peripherally acting agents (losartan and enalapril) were the most effective, showing restoration of the integrity of most of the myelin sheaths and the average Schwann cell number (Fig. 6E, F). Centrally acting agents (telmisartan and ramipril) showed some wavy myelin sheaths, but without hemorrhage (Fig. 6G, H).
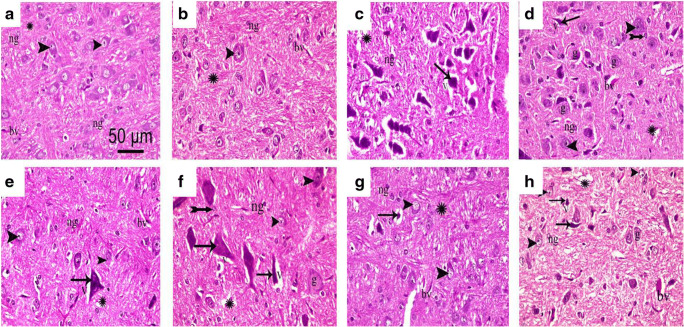
Effect on the Brainstem
Brainstem sections of both normal and sham groups subjected to H&E staining displayed normal morphologic features of neurons with vesicular nuclei and basophilic cytoplasm (Fig. 7A, B). CCI sections (Fig. 7C) showed affected neurons with shrunken, dark-stained nuclei surrounded by a pericellular halo, indicative of apoptosis. Besides, the neuropils were vacuolated and contained neuroglial cells with congested blood vessels. Sections obtained from rats treated with pregabalin showed some normal neurons with basophilic cytoplasm and others affected with dark-stained nuclei and pericellular halo. The acidophilic neuropil contained blood vessels with wide perivascular spaces and neuroglial cells. A large number of ghost cells were seen (Fig. 7D). Sections obtained from losartan-, enalapril-, telmisartan-, and ramipril-treated groups showed better neuronal architecture to varying degrees (Fig. 7E–H). Interestingly, centrally acting drugs (telmisartan and ramipril) displayed less affected neurons, as shown in Fig. 7G, H, respectively. Moreover, ghost cells were not observed in telmisartan-treated sections. Interestingly, the peripherally acting ARB (losartan) also showed more normal neurons (Fig. 7E) than the peripherally acting ACE inhibitor (enalapril).
Fig. 7.
Representative photomicrograph (H&E, × 400) of brainstem sections from different groups: (A) normal, (B) sham, (C) CCI, (D) pregabalin, (E) losartan, (F) enalapril, (G) telmisartan, and (H) ramipril. Arrowhead, arrow, bifid arrow, and asterisk (*) illustrate normal neurons, affected neurons with dark-stained cytoplasm and nuclei, perineural glial cell nuclei, and neuropil, respectively. N = Nissl granule; n = nuclei; bv = normal blood vessel; bva = dilated congested blood vessel; h = halo; A = astrocyte; O = oligodendrocytes; m = microglial cells. Scale bar, 50 μm
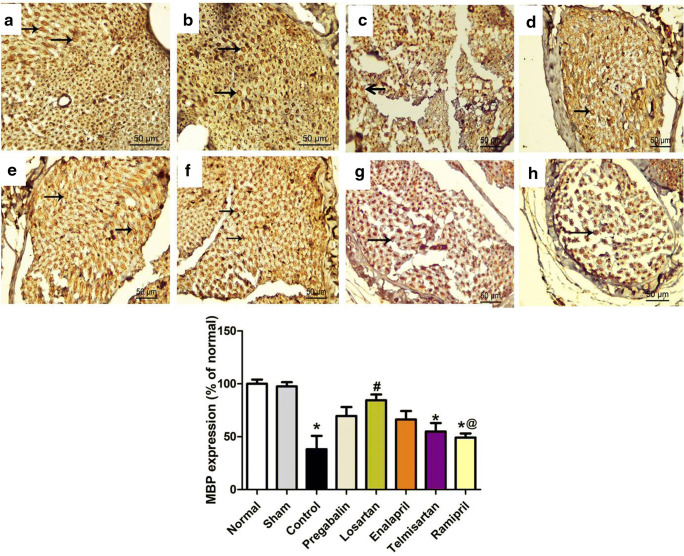
Sciatic Nerve MBP Expression
To investigate the extent of demyelination in the sciatic nerves of CCI rats, the expression of MBP, a major constituent of myelin sheath, was stained using immunohistochemistry (dark brown staining). Sciatic nerve sections of normal and sham rats showed a normal number of myelinated fibers and regular pattern of myelin organization (Fig. 8A, B). On the other hand, sciatic nerve sections of CCI rats showed an obvious loss of myelin, represented as reduced MPB expression in nerve fibers with the appearance of more interfiber spaces compared with those in the sham group (Fig. 8C). Sciatic nerves from rats treated with the peripherally acting RAAS drugs (losartan and enalapril) showed an increase in the average intensity of MBP staining that was comparable with that observed in the pregabalin-treated group (Fig. 8).
Fig. 8.
Photomicrographs of sciatic nerve transverse sections from different groups stained with MBP: (A) normal, (B) sham, (C) CCI, (D) pregabalin, (E) losartan, (F) enalapril, (G) telmisartan, and (H) ramipril. Lower panel represents the percentage of MBP immunoreactivity relative to the normal group. Data are presented as mean values ± SEM. (n = 5). *p < 0.05 versus sham group; #p < 0.05 versus CCI group; @p < 0.05 versus losartan group. Scale bar, 50 μm × 400
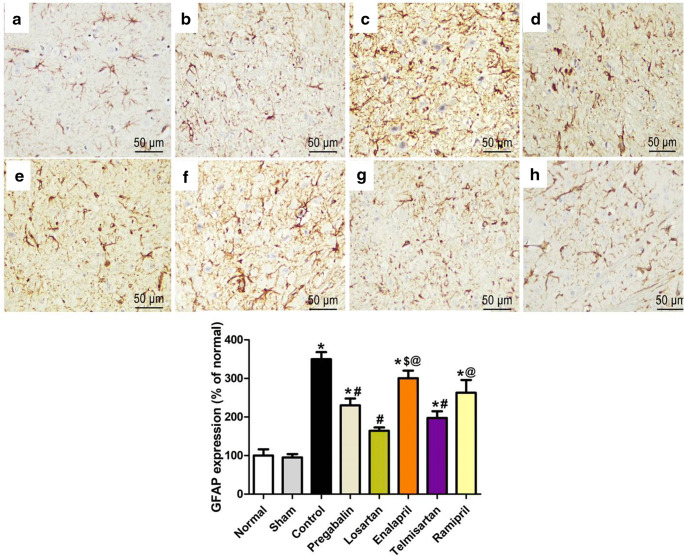
Brainstem GFAP Expression
The brainstem sections were immunohistochemically stained with the anti-GFAP antibody to elucidate the response of astrocytes to the neural damage in the different studied groups. Normal and sham groups showed GFAP-positive staining of astrocytes that exhibit few short and thin processes (Fig. 9A, B). On the other hand, sections from the CCI group showed abundant GFAP-positive staining with a significant increase in the number, thickness, and length of astrocyte processes (Fig. 9C). Sections from rats that received different treatments showed a reduced GFAP expression to variable degrees compared with that of the CCI group. Notably, ARBs (telmisartan and losartan) were more effective compared with ACE-Is (ramipril and enalapril) in reducing GFAP levels (Fig. 9E–H).
Fig. 9.
Photomicrographs of brainstem sections from different groups stained with GFAP: (A) normal, (B) sham, (C) CCI, (D) pregabalin, (E) losartan, (F) enalapril, (G) telmisartan, and (H) ramipril. Lower panel represents percentage of GFAP-immunoreactive structures relative to the normal group. Data are presented as mean values ± SEM. (n = 5). *p < 0.05 versus sham group; #p < 0.05 versus CCI group; @p < 0.05 versus losartan group. Scale bar, 50 μm × 400
Discussion
The present study was established to investigate the potential of using different RAAS-modifying drugs for treating peripheral NP and to test whether ARBs are superior to ACE-Is or vice versa in a CCI model of NP. This work also evaluated whether centrally acting RAAS drugs are preferable to peripherally acting ones in the same model. Our most important findings are as follows: 1) CCI rats showed marked increases in mechanical allodynia, heat hyperalgesia, mechanical hyperalgesia, and cold allodynia when measured at days 7 and 14 post; 2) CCI rats showed increases in the levels of inflammatory mediators (NF-Kβ, TNF-α, PGE2, and COX-2), (iNOS), phosphorylated P38 MAPK, STAT-3, ACE, AT1R, AT2R, and serum bradykinin along with oxidative stress in the sciatic nerve and brainstem; 3) diffirent RAAS treatments improved the behavioral and biochemical changes to varying degrees, with ARBs showing superiority over ACE-Is; 4) centrally acting ARB (telmisartan) showed a superior response for improving the behavioral changes, whereas a peripherally acting ARB (losartan) was more effective regarding the results of most biochemical measurements.
The CCI model is the most commonly employed neuropathic animal model of nerve damage that is associated with painful responses including allodynia and hyperalgesia [27, 49]. It has both peripheral nerve injury and inflammatory components, which can mimic NP in humans [25, 26]. Moreover, this model, compared with other models of peripheral neuropathy, resulted in the most sustained and generalized behavioral alterations [50].
Sciatic nerve ligation is associated with morphological and pathophysiological alterations of the injured nerve, along with pain-related behaviors, that peak at approximately 14 days postinjury [46, 51, 52]. In our study, CCI led to the development of cold allodynia, mechanical hyperalgesia, heat hyperalgesia, and mechanical dynamic allodynia when assessed on the 7th and 14th days after surgery. The administration of telmisartan, losartan, ramipril, or enalapril for 14 days significantly attenuated chronic constriction injury-induced behavioral alterations to different degrees (Fig. 1A–D). The current findings are supported by other studies that investigated the effects of telmisartan [9], ramipril [44], and losartan [42] in alleviating neuropathic and other pain stimuli. Moreover, our results revealed that ARBs are superior to ACE-Is and that the centrally acting ARB telmisartan is the most effective in attenuating the behavioral changes associated with CCI among all of the tested drugs. The high efficacy of telmisartan in this model is not surprising for a number of reasons. First, it has been reported to possess the highest affinity for the angiotensin AT1 receptors [53] and to have the most intense anti-inflammatory effects among angiotensin AT1 R antagonists [54]. Second, ARB can reach therapeutically relevant concentrations in the brain following peripheral administration (1-30 mg/kg) in rats [37, 55, 56]. This can explain its superiority in attenuating behavioral symptoms via central pain modulation.
To assess the role of drugs acting on RAAS in the neuroinflammatory part of this model system, we measured a number of inflammatory mediators known to be involved in the development of NP, such as NF-кB, PGE2, bradykinin, different cytokines, ROS [3], iNOS [57], JAK2/STAT-3, and P38 MAPK [14, 16]. Moreover, we measured the sciatic nerve MBP expression, which is highly expressed in the myelin sheaths and indicative of myelination [58]. Finally, the brainstem’s astrocyte activation, which is linked to neuronal toxicity and injury and a critical component for maintaining neuropathic pain [24, 59], was also evaluated utilizing the astrocyte marker GFAP. In the current study, the CCI model resulted in increased levels of NF-кB, iNOS, P38 MAPK phosphorylation, and STAT3 activation with a subsequent imbalance in the inflammatory cytokines (increases in TNF-α, PGE2, COX-2, and bradykinin) and oxidative stress (enhanced NOX and decreased catalase) in both sciatic nerve and brainstem tissues (Figs. 2, 3 and 4). These changes were associated with histopathological abnormalities in both sciatic nerve and brainstem (Figs. 6 and 7). Moreover, CCI leads to a marked sciatic nerve demyelination (decreased MBP expression) and the brainstem’s astrocyte activation (increased GFAP expression) (Figs. 8 and 9). On the other hand, AT1R blockade and, to a lesser extent, ACE inhibition for 14 days mitigated previously described inflammatory responses and histological changes in both tissue preparations (Figs. 2–9). Similar to the behavioral component, ARBs (losartan and telmisartan) were superior to ACE-Is (enalapril and ramipril) at alleviating the inflammatory component in this model. Moreover, ACE inhibitors failed to decrease the serum level of bradykinin (Fig. 3F), an effect that was reasonable, as these drugs are well known for increasing the level of bradykinin, one of the substrates of ACE, by inhibiting its breakdown [60]. In our model of peripheral neuropathy, the ability of the peripherally acting ACE-I enalapril to attenuate central inflammation is more likely due its effect on mitigating the sciatic nerve injury and the subsequent inflammatory cascade, which triggers the central component of the disease [61]. Alternatively, the drug may reach the CNS in an appreciable concentration to achieve central effects following chronic administration, especially with a disrupted BBB as a result of peripheral neuropathy [62, 63].
In line with our experiment, numerous studies have shown that COX2 and PGE2 levels are increased in NP [17, 64] through NF-KB activation [65]. Whereas ARBs in our study could decrease the levels of both COX2 and PGE2, ACE inhibitors achieved levels that were not significantly different from those in the CCI group (Fig. 3C, D). These findings could be a result of the accumulation of bradykinin, which can induce COX2 and hence PGE2 in different cell types [66–69]. Moreover, CCI-induced NP was also associated with a rise in iNOS level in both the sciatic nerve and brainstem. The role of iNOS has been well documented in peripheral [57] as well as central sensitization in NP [70]. However, in the present study, the administration of ARBs but not ACE-Is was successful at controlling the levels of iNOS both at the central and peripheral levels. These findings are supported by the reported increases of COX-2 and iNOS after bradykinin stimulation in myocardial cells [71]. The previous findings may explain in part the inferiority of ACE inhibitors in terms of behavioral, biochemical, and histopathological results.
Notably, losartan was superior to telmisartan in the later responses in both peripheral and central levels. This could be explained as follows: 1) The reported ability of ARBs to exert a central neuroprotective effect by their ability to maintain the BBB functionality, and hence indirectly attenuate central inflammation [72]; 2) losartan has AT1R-independent activity primarily related to its anti-inflammatory mechanisms, which are not shared by other ARBs [73]; and 3) although losartan exerts less lipophilicity than telmisartan and hence less permeability through the BBB [74], the disruption of BBB integrity as a result of peripheral neuropathy [62, 63] and the chronic administration of the drug may increase the central bioavailability of losartan.
JAK2/STAT-3 and P38 MAPK pathways are known to be activated in spinal cord microglia after peripheral nerve injury and contribute to the development of NP in rats [13, 14, 16]. Although this pathway has been reported to be involved in both the pathophysiology of NP and the neuronal inflammatory response of AngII [23], the role of this pathway in the protective effects exerted by RAAS blockers in this model system has yet to be fully investigated. Our data suggest that drugs acting on RAAS can downregulate the STAT-3/P38 MAPK pathway in the CCI model and hence exert a beneficial effect in NP modulation. Inhibitors of JAK-STAT3 signaling attenuate pain-related behavior in NP via suppressing astrocyte proliferation (GFAP expression) [24]. In line with this, losartan, which achieved the most beneficial effect in this model, had the lowest levels of phosphorylated STAT-3/P38-MAPK and GFAP among the treatments (Figs. 4 and 9).
The different components of the RAAS including AT1R, AT2R, and ACE were reported to be expressed in the nervous tissues, including the brainstem and sciatic nerve [75–77]. In the current study, CCI was associated with RAAS overactivation as evidenced by the increases in ACE level and AT1R expression (proinflammatory and pro-oxidant components of RAAS) in both brainstem and sciatic nerve tissues (Fig. 5A, B). This might have been due to the activation of NFкB and STAT3, which are key signaling molecules involved in RAAS overactivation [78, 79]. In line with this observation, previous studies reported the critical role of the renin–angiotensin system in pain modulation [10] and that the upregulation of ACE level and AT1R expression was involved in inflammation and pain [80, 81]. Interestingly, higher levels of AT2R were observed in our study. Although AT2R is known as the neuroprotective component in the RAAS axis [82, 83], its role in pain is controversial [84]. Our results are in agreement with previous studies that reported the upregulation of AT2R expression in the skin of neonatal rats following injury [85], and in cultured DRG neurons by estrogen to promote nociceptor axon sprouting [86]. In this model of peripheral neuropathy, RAAS-modulating drugs reduced AT1R expression and ACE levels along with reductions in AT2R expression in CCI rats (Fig. 5). The superiority of ARBs over ACE-Is regarding neuroprotective effects via AT2R upregulation was reported in retinal inflammation [87] and LPS-induced neuroinflammation [88]. However, our results were not in accordance with those findings, as both ACE-Is and ARBs in this study decreased the AT2R level both centrally (brainstem) and peripherally (sciatic nerve). This observation could be explained by their ability to decrease NFкB levels and inhibit the activation of STAT3, which are both involved in RAAS overactivation as discussed above. Notably, the blockade of AT2R appears to be promising in the treatment of neuropathy [89]. Recent studies reported the analgesic effects of small-molecule AT2R antagonists in peripheral nerve injury and antiretroviral NP models in rats [90, 91]. Interestingly, the non-RAAS-modulating drug pregabalin was also able to modify AT1 and AT2 receptor expression and ACE levels in our model. This effect is more likely due to its anti-inflammatory and analgesic potential [92] rather than a direct RAAS-modulating effect.
Taken together, the findings from the present study suggest the superiority of ARBs (peripheral and central) over ACE-Is in terms of the behavioral, biochemical, and histological changes associated with CCI. These findings are consistent with others demonstrating better neuroprotective and anti-inflammatory roles of an ARB (candesartan) when compared with an ACE-I (perindopril) via inhibition of the NF-KB and STAT-3 pathway in a model of neuroinflammation induced by lipopolysaccharide (LPS) [88]. The findings also match those of others who reported the superiority of ARBs over ACE-Is in a number of neurological disorders, including stroke [93], Alzheimer’s disease [94], traumatic brain injury [95], and Parkinson’s disease [96]. The idea that ARB neuroprotection not only is dependent upon the direct penetration of brain cells but could also be the result of indirect mechanisms on peripheral organs as well as the integrity of the blood–brain barrier [97] explains at least in part the similarity between losartan (peripherally acting agent) and telmisartan in some parts of the neuroinflammatory component in this model.
Unlike our findings, in a previous study, it was concluded that ACE-Is were superior to ARBs regarding neuroprotective and antioxidant effects in a model of diabetic peripheral neuropathy [98]. This conflict may be simply explained by the variant pathophysiologies of peripheral NP and diabetic NP. RAAS-modulating drugs ameliorate diabetic vascular dysfunction and hence neuropathy [99].
The regulation of AT1R and AT2R expression in brainstem and sciatic nerve tissues after CCI and the role of angiotensin after nerve injury warrant further study.
Conclusion
Our study has demonstrated significant anti-inflammatory and analgesic effects of different RAAS treatments in an animal model of peripheral neuropathy. This study provides evidence for the superiority of ARBs (peripherally and centrally acting) over ACE-Is in ameliorating the inflammatory and behavioral components of this model. Among the ARBs used, the centrally acting ARB telmisartan had a favorable effect in improving the behavioral component, whereas losartan was the most effective in both the biochemical and the histological results of NP. The previous findings could be attributed to 1) the superiority of ARBs in attenuating serum bradykinin, COX2, PGE2, and iNOS both centrally and peripherally and 2) the higher ability of ARBs to downregulate NF-KB, P38 MAPK, and STAT3 and to balance ACE, AT1R, and AT2R levels compared with ACE-Is. Finally, the study suggests that ARBs are a promising treatment approach to relieve neuropathic pain induced by a peripheral nerve trauma.
The Study Limitations
The current data support that ARBs have greater anti-inflammatory and analgesic effects compared to ACE-Is in a CCI model of NP; however, the comparisons were performed with only 1 dose of each drug. Further research utilizing a dose-response design to demonstrate whether or not an increase in the dose of each individual drug correlates with an increase in the antihyperalgesic and anti-inflammatory activity in this model is needed.
Electronic Supplementary Material
(PDF 453 kb)
Acknowledgments
This work did not receive any funding from funding agencies in the public, commercial, or not-for-profit sectors. The authors would like to thank Dr. Amira Alsemeh (Anatomy and Embryology Department, Faculty of Medicine, Zagazig University, Egypt) for performing the immunohistochemistry scoring and analysis.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Abbreviations
- ACE-I
Angiotensin-converting enzyme inhibitor
- Ang II
Angiotensin II
- ARB
AT1 receptor blocker
- AT1R
Angiotensin II type 1 receptor
- AT2R
Angiotensin II type 2 receptor
- BBB
Blood–brain barrier
- MBP
Myelin basic protein
- CCI
Chronic constriction injury
- COX-2
Cyclooxygenase-2
- DRG
Dorsal root ganglia
- ELISA
Enzyme-linked immunosorbent assay
- GFAP
Glial fibrillary acidic protein
- H&E
Hematoxylin and eosin
- iNOS
Inducible nitric oxide synthase
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- LPS
Lipopolysaccharide
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NFκB
Nuclear factor-kappa B
- NP
Neuropathic pain
- PGE2
Prostaglandin E2
- P38 MAPK
P38 mitogen activated protein kinase
- RAAS
Renin–angiotensin–aldosterone system
- ROS
Reactive oxygen species
- STAT3
Signal transducer and activator of transcription 3
- TNF-α
Tumor necrosis factor α
Authors’ Contributions
Participated in research design: Rezq, Hegazy, and Fahmy; conducted experiments: Hegazy and Rezq; performed data analysis: Hegazy and Rezq; contributed to the writing of the manuscript: Hegazy, Rezq, and Fahmy.
Data Availability
Access to the full dataset used in this work will be granted upon request to the lead author.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflicts of interest.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: 10.1007/s13311-023-01449-2
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nora Hegazy and Samar Rezq contributed equally to this work.
Change history
10/10/2023
This article has been retracted. Please see the Retraction Notice for more detail: 10.1007/s13311-023-01449-2
References
- 1.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002. doi: 10.1038/nrdp.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. 2014;348:f7656. doi: 10.1136/bmj.f7656. [DOI] [PubMed] [Google Scholar]
- 4.Harden N, Cohen M. Unmet needs in the management of neuropathic pain. J Pain Symptom Manage. 2003;25(5 Suppl):S12–7. doi: 10.1016/S0885-3924(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 5.Roldan CJ, Song J, Engle MP, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers modulate the function of myelinated fibers after chemotherapy: a quantitative sensory testing study. Pain Physician. 2017;20(4):281–292. doi: 10.36076/ppj.2017.292. [DOI] [PubMed] [Google Scholar]
- 6.Zhuo JL, Li XC. New insights and perspectives on intrarenal renin-angiotensin system: focus on intracrine/intracellular angiotensin II. Peptides. 2011;32(7):1551–65. doi: 10.1016/j.peptides.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bessaguet F, Magy L, Desmouliere A, Demiot C. The therapeutic potential of renin angiotensin aldosterone system (RAAS) in chronic pain: from preclinical studies to clinical trials. Expert Rev Neurother. 2016;16(3):331–339. doi: 10.1586/14737175.2016.1150179. [DOI] [PubMed] [Google Scholar]
- 8.Khan N, Bakshi KS, Jaggi AS, Singh N. Ameliorative potential of spironolactone in diabetes induced hyperalgesia in mice. Yakugaku Zasshi. 2009;129(5):593–599. doi: 10.1248/yakushi.129.593. [DOI] [PubMed] [Google Scholar]
- 9.Jaggi AS, Singh N. Exploring the potential of telmisartan in chronic constriction injury-induced neuropathic pain in rats. Eur J Pharmacol. 2011;667(1-3):215–221. doi: 10.1016/j.ejphar.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Bali A, Singh N, Jaggi AS. Renin-angiotensin system in pain: existing in a double life? J Renin Angiotensin Aldosterone Syst. 2014;15(4):329–340. doi: 10.1177/1470320313503694. [DOI] [PubMed] [Google Scholar]
- 11.Iwanami J, Mogi M, Tsukuda K, et al. Low dose of telmisartan prevents ischemic brain damage with peroxisome proliferator-activated receptor-gamma activation in diabetic mice. J Hypertens. 2010;28(8):1730–1777. doi: 10.1097/HJH.0b013e32833a551a. [DOI] [PubMed] [Google Scholar]
- 12.Sagawa K, Nagatani K, Komagata Y, Yamamoto K. Angiotensin receptor blockers suppress antigen-specific T cell responses and ameliorate collagen-induced arthritis in mice. Arthritis Rheum. 2005;52(6):1920–1928. doi: 10.1002/art.21040. [DOI] [PubMed] [Google Scholar]
- 13.Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23(7):2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kandalam U, Clark MA. Angiotensin II activates JAK2/STAT3 pathway and induces interleukin-6 production in cultured rat brainstem astrocytes. Regul Pept. 2010;159(1-3):110–116. doi: 10.1016/j.regpep.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Dominguez E, Rivat C, Pommier B, Mauborgne A, Pohl M. JAK/STAT3 pathway is activated in spinal cord microglia after peripheral nerve injury and contributes to neuropathic pain development in rat. J Neurochem. 2008;107(1):50–60. doi: 10.1111/j.1471-4159.2008.05566.x. [DOI] [PubMed] [Google Scholar]
- 17.Ma W, Quirion R. Does COX2-dependent PGE2 play a role in neuropathic pain? Neurosci Lett. 2008;437(3):165–169. doi: 10.1016/j.neulet.2008.02.072. [DOI] [PubMed] [Google Scholar]
- 18.Jaimes EA, Tian RX, Pearse D, Raij L. Up-regulation of glomerular COX-2 by angiotensin II: role of reactive oxygen species. Kidney Int. 2005;68(5):2143–2153. doi: 10.1111/j.1523-1755.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 19.Bataller R, Gäbele E, Schoonhoven R, et al. Prolonged infusion of angiotensin II into normal rats induces stellate cell activation and proinflammatory events in liver. Am J Physiol Gastrointest Liver Physiol. 2003;285(3):G642–G651. doi: 10.1152/ajpgi.00037.2003. [DOI] [PubMed] [Google Scholar]
- 20.Recinos A, 3rd, Lejeune WS, Sun H, et al. Angiotensin II induces IL-6 expression and the Jak-STAT3 pathway in aortic adventitia of LDL receptor-deficient mice. Atherosclerosis. 2007;194(1):125–133. doi: 10.1016/j.atherosclerosis.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang YM, Ma Y, Zheng JP, et al. Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res. 2009;82(3):503–512. doi: 10.1093/cvr/cvp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rojas M, Zhang W, Lee D, et al. Role of IL-6 in angiotensin II-induced retinal vascular inflammation. Invest Ophthalmol Vis Sci. 2010;51(3):1709–1718. doi: 10.1167/iovs.09-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark MA, Gonzalez N. Angiotensin II stimulates rat astrocyte mitogen-activated protein kinase activity and growth through EGF and PDGF receptor transactivation. Regul Pept. 2007;144(1-3):115–122. doi: 10.1016/j.regpep.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Tsuda M, Khoro Y, Yano T, et al. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain. 2011;134(Pt 4):1127–1139. doi: 10.1093/brain/awr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PJ, A Wu, and G Moalem-Taylor, Chronic constriction of the sciatic nerve and pain hypersensitivity testing in rats. J Vis Exp, 2012(61). [DOI] [PMC free article] [PubMed]
- 26.De Vry J, Kuhl E, Franken-Kunkel P, Eckel G. Pharmacological characterization of the chronic constriction injury model of neuropathic pain. Eur J Pharmacol. 2004;491(2-3):137–148. doi: 10.1016/j.ejphar.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 27.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 28.Flatters SJ, Bennett GJ. Ethosuximide reverses paclitaxel-and vincristine-induced painful peripheral neuropathy. Pain. 2004;109(1-2):150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 29.Jain V, Jaggi AS, Singh N. Ameliorative potential of rosiglitazone in tibial and sural nerve transection-induced painful neuropathy in rats. Pharmacol Res. 2009;59(6):385–392. doi: 10.1016/j.phrs.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Caterina MJ, Leffler A, Malmberg AB, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 31.Erichsen HK, Blackburn-Munro G. Pharmacological characterisation of the spared nerve injury model of neuropathic pain. Pain. 2002;98(1-2):151–161. doi: 10.1016/S0304-3959(02)00039-8. [DOI] [PubMed] [Google Scholar]
- 32.Thibault K, Bonnard E, Dubacq S, et al. Antinociceptive and anti-allodynic effects of oral PL37, a complete inhibitor of enkephalin-catabolizing enzymes, in a rat model of peripheral neuropathic pain induced by vincristine. Eur J Pharmacol. 2008;600(1-3):71–77. doi: 10.1016/j.ejphar.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Weissman-Fogel I, Dashkovsky A, Rogowski Z, Yarnitsky D. Vagal damage enhances polyneuropathy pain: additive effect of two algogenic mechanisms. Pain. 2008;138(1):153–162. doi: 10.1016/j.pain.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Yamada K, Uchida S, Takahashi S, et al. Effect of a centrally active angiotensin-converting enzyme inhibitor, perindopril, on cognitive performance in a mouse model of Alzheimer's disease. Brain Res. 2010;1352:176–186. doi: 10.1016/j.brainres.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Jiang L, Zhu R, Bu Q, et al. Brain renin-angiotensin system blockade attenuates methamphetamine-induced hyperlocomotion and neurotoxicity. Neurotherapeutics. 2018;15(2):500–510. doi: 10.1007/s13311-018-0613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gohlke P, Scholkens B, Henning R, Urbach H, Unger T, et al. Inhibition of converting enzyme in brain tissue and cerebrospinal fluid of rats following chronic oral treatment with the converting enzyme inhibitors ramipril and Hoe 288. J Cardiovasc Pharmacol. 1989;14(Suppl 4):S32–S36. doi: 10.1097/00005344-198900000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Gohlke P, Weiss S, Jansen A, et al. AT1 receptor antagonist telmisartan administered peripherally inhibits central responses to angiotensin II in conscious rats. J Pharmacol Exp Ther. 2001;298(1):62–70. [PubMed] [Google Scholar]
- 38.van Meel JC, Redemann N, Haigh RM. Hypotensive effects of the angiotensin II antagonist telmisartan in conscious chronically-instrumented transgenic rats. Arzneimittelforschung. 1996;46(8):755–759. [PubMed] [Google Scholar]
- 39.Piepho RW. Overview of the angiotensin-converting-enzyme inhibitors. Am J Health Syst Pharm. 2000;57(Suppl 1):S3–S7. doi: 10.1093/ajhp/57.suppl_1.S3. [DOI] [PubMed] [Google Scholar]
- 40.Brown NJ, Vaughan DE. Angiotensin-converting enzyme inhibitors. Circulation. 1998;97(14):1411–1420. doi: 10.1161/01.CIR.97.14.1411. [DOI] [PubMed] [Google Scholar]
- 41.Holenarsipur VK, Gaud N, Sinha J, et al. Absorption and cleavage of enalapril, a carboxyl ester prodrug, in the rat intestine: in vitro, in situ intestinal perfusion and portal vein cannulation models. Biopharm Drug Dispos. 2015;36(6):385–397. doi: 10.1002/bdd.1950. [DOI] [PubMed] [Google Scholar]
- 42.Kim E, Hwang SH, Kim HK, Abdi S, Kim HK. Losartan, an angiotensin II type 1 receptor antagonist, alleviates mechanical hyperalgesia in a rat model of chemotherapy-induced neuropathic pain by inhibiting inflammatory cytokines in the dorsal root ganglia. Mol Neurobiol. 2019;56(11):7408–7419. doi: 10.1007/s12035-019-1616-0. [DOI] [PubMed] [Google Scholar]
- 43.Wagner J, Drab M, Bohlender J, Amman K, Wienen W, Ganten D. Effects of AT1 receptor blockade on blood pressure and the renin-angiotensin system in spontaneously hypertensive rats of the stroke prone strain. Clin Exp Hypertens. 1998;20(2):205–221. doi: 10.3109/10641969809053215. [DOI] [PubMed] [Google Scholar]
- 44.Kaur P, Muthuraman A, Kaur J. Ameliorative potential of angiotensin-converting enzyme inhibitor (ramipril) on chronic constriction injury of sciatic nerve induced neuropathic pain in mice. J Renin Angiotensin Aldosterone Syst. 2015;16(1):103–112. doi: 10.1177/1470320314556171. [DOI] [PubMed] [Google Scholar]
- 45.Al-Rejaie SS, Abuohashish HM, Ahmed MM, Arrejaie AS, Aleisa AM, Alsharari SD. Telmisartan inhibits hyperalgesia and inflammatory progression in a diabetic neuropathic pain model of Wistar rats. Neurosciences. 2015;20(2):115. doi: 10.17712/nsj.2015.2.20140511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kukkar A, Singh N, Jaggi AS. Attenuation of neuropathic pain by sodium butyrate in an experimental model of chronic constriction injury in rats. J Formos Med Assoc. 2014;113(12):921–928. doi: 10.1016/j.jfma.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Mills EP, Dipietro F, Peck AZ, Murray GM, Vickers ER, Henderson LA. Brainstem pain-control circuitry connectivity in chronic neuropathic pain. J Neurosci. 2018;38(2):465–473. doi: 10.1523/JNEUROSCI.1647-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sen O, Sayilgan NC, Tutuncu AC, Bakan M, Koksal GM, Oz H. Evaluation of sciatic nerve damage following intraneural injection of bupivacaine, levobupivacaine and lidocaine in rats. Braz J Anesthesiol. 2016;66(3):272–275. doi: 10.1016/j.bjan.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 49.Jaggi AS, Singh N. Therapeutic targets for the management of peripheral nerve injury-induced neuropathic pain. CNS Neurol Disord Drug Targets. 2011;10(5):589–609. doi: 10.2174/187152711796235041. [DOI] [PubMed] [Google Scholar]
- 50.Dowdall T, Robinson I, Meert TF. Comparison of five different rat models of peripheral nerve injury. Pharmacol Biochem Behav. 2005;80(1):93–108. doi: 10.1016/j.pbb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 51.Jaggi AS, Singh N. Differential effect of spironolactone in chronic constriction injury and vincristine-induced neuropathic pain in rats. Eur J Pharmacol. 2010;648(1-3):102–109. doi: 10.1016/j.ejphar.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 52.Jiang Y-Q, Xing GG, Wang SL, et al. Axonal accumulation of hyperpolarization-activated cyclic nucleotide-gated cation channels contributes to mechanical allodynia after peripheral nerve injury in rat. Pain. 2008;137(3):495–506. doi: 10.1016/j.pain.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 53.Kakuta H, Sudoh K, Sasamata M, Yamagishi S. Telmisartan has the strongest binding affinity to angiotensin II type 1 receptor: comparison with other angiotensin II type 1 receptor blockers. Int J Clin Pharmacol Res. 2005;25(1):41–46. [PubMed] [Google Scholar]
- 54.Kurtz TW, Pravenec M. Antidiabetic mechanisms of angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists: beyond the renin–angiotensin system. J Hypertens. 2004;22(12):2253–2261. doi: 10.1097/00004872-200412000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Noda A, Fushiki H, Murakami Y, et al. Brain penetration of telmisartan, a unique centrally acting angiotensin II type 1 receptor blocker, studied by PET in conscious rhesus macaques. Nucl Med Biol. 2012;39(8):1232–1235. doi: 10.1016/j.nucmedbio.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 56.Shimizu K, Takashima T, Yamani T, et al. Whole-body distribution and radiation dosimetry of [11C] telmisartan as a biomarker for hepatic organic anion transporting polypeptide (OATP) 1B3. Nucl Med Biol. 2012;39(6):847–853. doi: 10.1016/j.nucmedbio.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 57.Levy D, Hoke A, Zochodne DW. Local expression of inducible nitric oxide synthase in an animal model of neuropathic pain. Neurosci Lett. 1999;260(3):207–209. doi: 10.1016/S0304-3940(98)00982-3. [DOI] [PubMed] [Google Scholar]
- 58.Giorgetti E, Obrecht M, Ronco M, et al. Magnetic resonance imaging as a biomarker in rodent peripheral nerve injury models reveals an age-related impairment of nerve regeneration. Sci Rep. 2019;9(1):13508. doi: 10.1038/s41598-019-49850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Callaghan JP, Sriram K. Glial fibrillary acidic protein and related glial proteins as biomarkers of neurotoxicity. Expert Opin Drug Saf. 2005;4(3):433–442. doi: 10.1517/14740338.4.3.433. [DOI] [PubMed] [Google Scholar]
- 60.Fox AJ, Lalloo UG, Belvisi MG, Bernareggi M, Chung KF, Barnes PJ, et al. Bradykinin–evoked sensitization of airway sensory nerves: a mechanism for ACE–inhibitor cough. Nat Med. 1996;2(7):814. doi: 10.1038/nm0796-814. [DOI] [PubMed] [Google Scholar]
- 61.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306(5944):686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 62.Beggs S, Liu XJ, Kwan C, Salter MW. Peripheral nerve injury and TRPV1-expressing primary afferent C-fibers cause opening of the blood-brain barrier. Mol Pain. 2010;6(1):74. doi: 10.1186/1744-8069-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huber J, Witt KA, Hom S, Egleton RD, Mark KS, Davis TP. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2001;280(3):H1241–H1248. doi: 10.1152/ajpheart.2001.280.3.H1241. [DOI] [PubMed] [Google Scholar]
- 64.Ma W, Eisenach JC. Morphological and pharmacological evidence for the role of peripheral prostaglandins in the pathogenesis of neuropathic pain. Eur J Neurosci. 2002;15(6):1037–1047. doi: 10.1046/j.1460-9568.2002.01940.x. [DOI] [PubMed] [Google Scholar]
- 65.Coronel MF, Labombarda F, De Nicola AF, Gonzalez SL. Progesterone reduces the expression of spinal cyclooxygenase-2 and inducible nitric oxide synthase and prevents allodynia in a rat model of central neuropathic pain. Eur J Pain. 2014;18(3):348–359. doi: 10.1002/j.1532-2149.2013.00376.x. [DOI] [PubMed] [Google Scholar]
- 66.Lu DY, Leung YM, Huang SM, Wong KL. Bradykinin-induced cell migration and COX-2 production mediated by the bradykinin B1 receptor in glioma cells. J Cell Biochem. 2010;110(1):141–150. doi: 10.1002/jcb.22520. [DOI] [PubMed] [Google Scholar]
- 67.Nie M, Pang L, Inoue H, Knox AJ. Transcriptional regulation of cyclooxygenase 2 by bradykinin and interleukin-1β in human airway smooth muscle cells: involvement of different promoter elements, transcription factors, and histone H4 acetylation. Mol Cell Biol. 2003;23(24):9233–9244. doi: 10.1128/MCB.23.24.9233-9244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodriguez JA, Vio CP, Pedraza PL, JC MG, Ferrerri NR. Bradykinin regulates cyclooxygenase-2 in rat renal thick ascending limb cells. Hypertension. 2004;44(2):230–235. doi: 10.1161/01.HYP.0000136751.04336.e9. [DOI] [PubMed] [Google Scholar]
- 69.Inoue A, Iwasa M, Nishikura Y, Ogawa S, Nakasuka A, Nakata Y. The long-term exposure of rat cultured dorsal root ganglion cells to bradykinin induced the release of prostaglandin E2 by the activation of cyclooxygenase-2. Neurosci Lett. 2006;401(3):242–247. doi: 10.1016/j.neulet.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 70.Martucci C, Trovato A, Costa B, et al. The purinergic antagonist PPADS reduces pain related behaviours and interleukin-1β, interleukin-6, iNOS and nNOS overproduction in central and peripheral nervous system after peripheral neuropathy in mice. Pain. 2008;137(1):81–95. doi: 10.1016/j.pain.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 71.Catalán M, Smolic C, Contreras A, et al. Differential regulation of collagen secretion by kinin receptors in cardiac fibroblast and myofibroblast. Toxicol Appl Pharmacol. 2012;261(3):300–308. doi: 10.1016/j.taap.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 72.Saavedra JM. Angiotensin II AT(1) receptor blockers as treatments for inflammatory brain disorders. Clin Sci (Lond) 2012;123(10):567–590. doi: 10.1042/CS20120078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sadoshima J, Novel AT1 receptor–independent functions of losartan, 2002, Am Heart Assoc. [DOI] [PubMed]
- 74.Takai S, Kirimura K, Jin D, et al. Significance of angiotensin II receptor blocker lipophilicities and their protective effect against vascular remodeling. Hypertens Res. 2005;28(7):593. doi: 10.1291/hypres.28.593. [DOI] [PubMed] [Google Scholar]
- 75.Chevillard C, Saavedra JM. Distribution of angiotensin-converting enzyme activity in specific areas of the rat brain stem. J Neurochem. 1982;38(1):281–284. doi: 10.1111/j.1471-4159.1982.tb10883.x. [DOI] [PubMed] [Google Scholar]
- 76.Chang AY, CHL F, Huang CW, et al. Interplay between brain stem angiotensins and monocyte chemoattractant protein-1 as a novel mechanism for pressor response after ischemic stroke. Neurobiol Dis. 2014;71:292–304. doi: 10.1016/j.nbd.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 77.Pavel J, Tang H, Brimijoin S, Moughamian A, et al. Expression and transport of angiotensin II AT1 receptors in spinal cord, dorsal root ganglia and sciatic nerve of the rat. Brain Res. 2008;1246:111–122. doi: 10.1016/j.brainres.2008.09.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davies NM, Kehoe PG, Ben-Shlomo Y, Martin RM. Associations of anti-hypertensive treatments with Alzheimer's disease, vascular dementia, and other dementias. J Alzheimers Dis. 2011;26(4):699–708. doi: 10.3233/JAD-2011-110347. [DOI] [PubMed] [Google Scholar]
- 79.Haack KK, Mitra AK, Zucker IH. NF-κB and CREB are required for angiotensin II type 1 receptor upregulation in neurons. Plos One. 2013;8(11):e78695. doi: 10.1371/journal.pone.0078695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yan K, Shen Y. Aliskiren has chondroprotective efficacy in a rat model of osteoarthritis through suppression of the local renin-angiotensin system. Mol Med Rep. 2017;16(4):3965–3973. doi: 10.3892/mmr.2017.7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang Y, Wei W. Angiotensin II in inflammation, immunity and rheumatoid arthritis. Clin Exp Immunol. 2015;179(2):137–145. doi: 10.1111/cei.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McCarthy CA, Vinh A, BRS B, Sobey CG, Callaway JK, Widdop RE. Angiotensin II type 2 receptor stimulation initiated after stroke causes neuroprotection in conscious rats. Hypertension. 2012;60(6):1531–1537. doi: 10.1161/HYPERTENSIONAHA.112.199646. [DOI] [PubMed] [Google Scholar]
- 83.McCarthy CA, Vinh A, Callaway JK, Widdop RE. Angiotensin AT2 receptor stimulation causes neuroprotection in a conscious rat model of stroke. Stroke. 2009;40(4):1482. doi: 10.1161/STROKEAHA.108.531509. [DOI] [PubMed] [Google Scholar]
- 84.Smith MT, Wyse BD, Edwards SR. Small molecule angiotensin II type 2 receptor (AT2R) antagonists as novel analgesics for neuropathic pain: comparative pharmacokinetics, radioligand binding, and efficacy in rats. Pain Medicine. 2013;14(5):692–705. doi: 10.1111/pme.12063. [DOI] [PubMed] [Google Scholar]
- 85.Viswanathan M, Saavedra JM. Expression of angiotensin II AT2 receptors in the rat skin during experimental wound healing. Peptides. 1992;13(4):783–786. doi: 10.1016/0196-9781(92)90187-8. [DOI] [PubMed] [Google Scholar]
- 86.Chakrabarty A, Blacklock A, Svojanovsky S, Smkith PG. Estrogen elicits dorsal root ganglion axon sprouting via a renin-angiotensin system. Endocrinology. 2008;149(7):3452–3460. doi: 10.1210/en.2008-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kurihara T, Ozawa K, Shinoda K, et al. Neuroprotective effects of angiotensin II type 1 receptor (AT1R) blocker, telmisartan, via modulating AT1R and AT2R signaling in retinal inflammation. Invest Ophthalmol Vis Sci. 2006;47(12):5545–5552. doi: 10.1167/iovs.06-0478. [DOI] [PubMed] [Google Scholar]
- 88.Bhat SA, Goel R, Shukla R, Hanif K. Angiotensin receptor blockade modulates NFκB and STAT3 signaling and inhibits glial activation and neuroinflammation better than angiotensin-converting enzyme inhibition. Mol Neurobiol. 2016;53(10):6950–6967. doi: 10.1007/s12035-015-9584-5. [DOI] [PubMed] [Google Scholar]
- 89.Salat K, Gryzlo B, Kulig K. Experimental drugs for neuropathic pain. Curr Neuropharmacol. 2018;16(8):1193–1209. doi: 10.2174/1570159X16666180510151241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anand U, Yiangou Y, Sinisi M, et al. Mechanisms underlying clinical efficacy of Angiotensin II type 2 receptor (AT 2 R) antagonist EMA401 in neuropathic pain: clinical tissue and in vitro studies. Mol Pain. 2015;11(1):38. doi: 10.1186/s12990-015-0038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hesselink JMK, Schatman ME. EMA401: an old antagonist of the AT2R for a new indication in neuropathic pain. J Pain Res. 2017;10:439. doi: 10.2147/JPR.S128520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abu-Rish EY, Mansour A, Mansour H, Dahabiyeh LA, Aleidi SM, Bustanji Y. Pregabalin inhibits in vivo and in vitro cytokine secretion and attenuates spleen inflammation in lipopolysaccharide/concanavalin A -induced murine models of inflammation. Sci Rep. 2020;10(1):4007. doi: 10.1038/s41598-020-61006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ando H, Zhou J, Macova M, Imboden H, Saavedra JM. Angiotensin II AT1 receptor blockade reverses pathological hypertrophy and inflammation in brain microvessels of spontaneously hypertensive rats. Stroke. 2004;35(7):1726–1731. doi: 10.1161/01.STR.0000129788.26346.18. [DOI] [PubMed] [Google Scholar]
- 94.Danielyan L, Klein R, Hanson LR, et al. Protective effects of intranasal losartan in the APP/PS1 transgenic mouse model of Alzheimer disease. Rejuvenation Res. 2010;13(2-3):195–201. doi: 10.1089/rej.2009.0944. [DOI] [PubMed] [Google Scholar]
- 95.Villapol S, Yaszemski AK, Logan TT, Sanchez-Lemus E, Saavedra JM, Symes AJ. Candesartan, an angiotensin II at 1-receptor blocker and PPAR-γ agonist, reduces lesion volume and improves motor and memory function after traumatic brain injury in mice. Neuropsychopharmacology. 2012;37(13):2817. doi: 10.1038/npp.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dominguez-Meijide A, Villar-Cheda B, Garrido-Gil P, Sierra-Paredes G, Guerra MJ, Labandeira-Garcia JL, et al. Effect of chronic treatment with angiotensin type 1 receptor antagonists on striatal dopamine levels in normal rats and in a rat model of Parkinson's disease treated with L-DOPA. Neuropharmacology. 2014;76:156–168. doi: 10.1016/j.neuropharm.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 97.Villapol S, Saavedra JM. Neuroprotective effects of angiotensin receptor blockers. Am J H. 2015;28(3):289–299. doi: 10.1093/ajh/hpu197. [DOI] [PubMed] [Google Scholar]
- 98.Coppey LJ, Davidson EP, Rinehart TW, et al. ACE inhibitor or angiotensin II receptor antagonist attenuates diabetic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2006;55(2):341–348. doi: 10.2337/diabetes.55.02.06.db05-0885. [DOI] [PubMed] [Google Scholar]
- 99.Malik RA, Can diabetic neuropathy be prevented by angiotensin-converting enzyme inhibitors?, 2000 Taylor & Francis [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 453 kb)
Data Availability Statement
Access to the full dataset used in this work will be granted upon request to the lead author.