Figure 1.
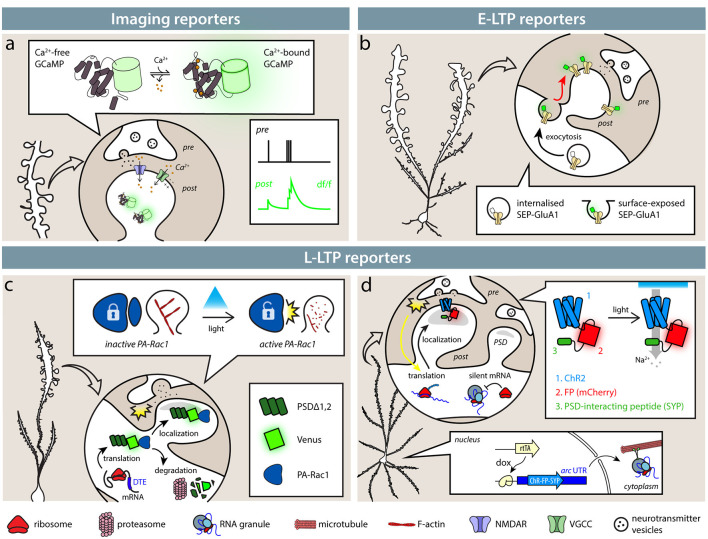
Available tools identify synapses based on their activity. (A) Imaging reporters (genetically encoded calcium indicators, GECIs), such as calcium (Ca2+) indicators, allow the experimenter to identify active synapses in live imaging. Ca2+ influx through NMDARs (blue) and VGCCs (green), as well as from intracellular stores, causes GECIs, such as GCaMP6s, to shift from a dark to a fluorescent state. Synaptic activity then results in an instantaneous change in fluorescence. (B) Exposure of SEP-tagged AMPA receptors (SEP-GluA1) labels synapses during E-LTP. In intracellular stores, SEP fluorescence is quenched by the acidic pH, so that only exposed AMPA receptors following synaptic activity are fluorescent. (C,D) SA-Ch and AS-PaRac1 reporters are expressed at synapses following potentiation induction. (C) AS-PaRac1 is expressed at synapses following potentiation thanks to Arc dendritic targeting element (DTE) in the 3′-UTR. PSDΔ1, 2 anchors the protein to the postsynaptic density (PSD) and promotes its degradation outside the synapse. AS-PaRac1 encodes a light-sensitive Rac1 form fused to Venus fluorescent protein. When it activated by blue light, it causes actin depolymerization and spine shrinkage. (D) SA-Ch encodes the ChR2 variant ChETA fused to the red fluorescent protein (RFP) mCherry and to SYP tag interacting with the PSD. Arc UTR sequences maintain the mRNA in repressed state and allow its translation at potentiated synapses. Like ChR2, SA-Ch is a cation channel that causes depolarizing photocurrents, but in principle, it could be substituted by opsins of other ionic specificities.