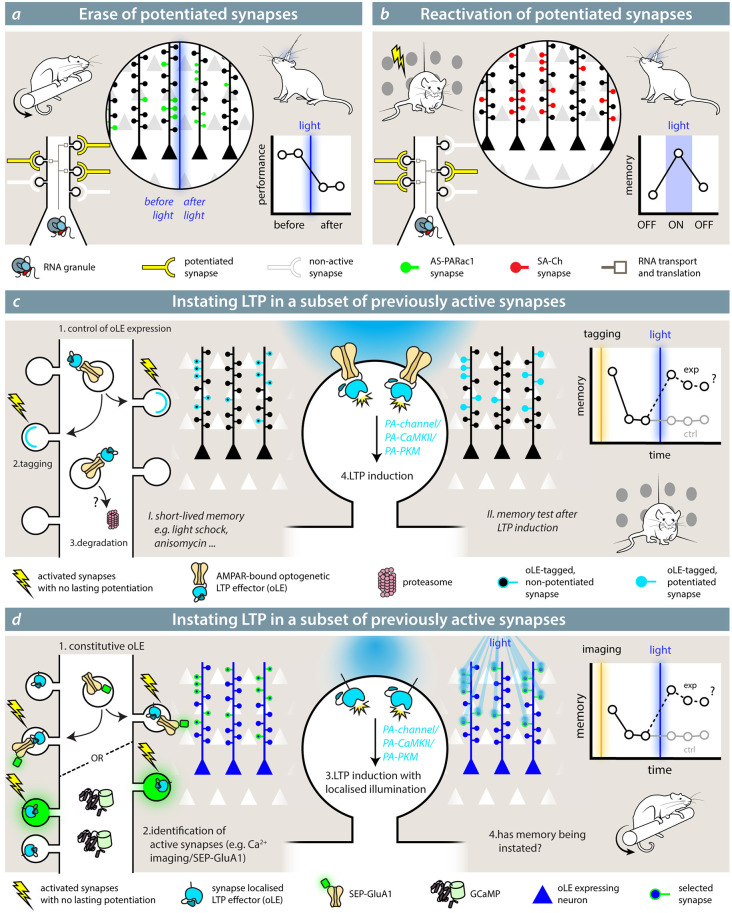
Figure 3.
Synaptic effectors in the study of memory. (A) Hayashi-Takagi et al. (2015) used the optogenetic reporter for synaptic potentiation AS-PARac1 to erase potentiated synapses by the learning of a motor memory task (rotarod). After learning, synapses express AS-PARac1. After blue-light illumination, labeled spines are shrunken, and the performance of the animal in the rotarod task is significantly reduced. (B) With SA-Ch (or any SA-Ch* opsin variant; Gobbo et al., 2017), it is possible to reactivate synapses that underwent potentiation during the learning (tagging) phase. If these play a role in the representation of the associated memory, re-activating them would elicit a coherent behavior during light illumination. While experiments outlined in (A,B) demonstrate the necessity role of potentiated synapses in the representation of a memory, theoretical experiments (C,D) are mimicry experiments aimed at demonstrating that potentiation of a set of synapses is sufficient for the formation and expression of a memory. The optogenetic LTP effector (oLE) could be based on either kinases that have been shown to have a prominent role in LTP, i.e., PKMζ or a constitutively active form of αCaMKII or light-sensitive NMDAR channels. To control their activity, a light-sensitive form of the two kinases has to be devised. The animal is first trained under conditions that do not form a long-lasting memory and/or impede potentiation; for example, a weak training, such as mild-shock contextual fear conditioning, anisomycin infusion, etc. In (C), the oLE localization to relevant synapses is achieved by fusing it to a GluA1 subunit, and it is coupled to AMPAR exposure. Control of expression would be critical, but specificity could be improved by increased degradation. If the interpretation of the role of LTP is correct, induction of LTP at this set of synapses would cause the formation of a memory. In (D), the opto-LTP effector is present at all synapses. The experimenter first detects active synapses by means of an imaging reporter (calcium imaging or visualization of SEP-GluA1, for example), then selectively activates the oLE at the selected synapses with patterned illumination.