Figure 6. Interference between C1 and C2.
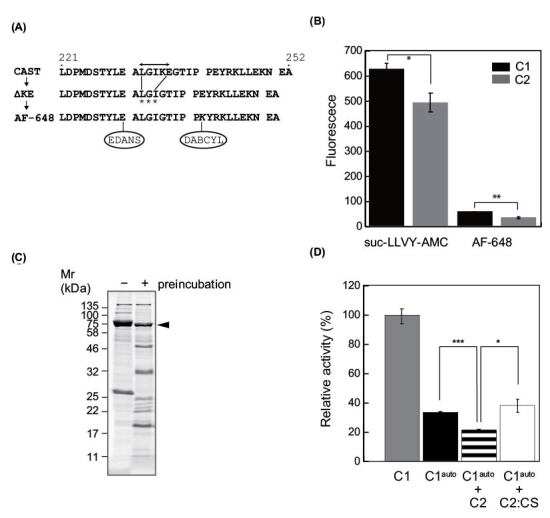
(A) Modification of the CAST sequence for FRET substrate. In an inhibitory domain B of rat CAST (221-252 of P27321), the sequence GIKEG escapes from the catalytic cleft of calpain (bidirectional arrow). Deletion of two amino acid residues, Lys235 and Glu236, results in proteolysis of CAST within the kink (ΔKE, asterisks) [18]. In a FRET substrate, AF-648, substitution of Glu232Lys (lower, bold italic) were introduced in addition to two amino acid residues (Lys235 and Glu236). (B) Activity assay of C1 and C2 using FRET substrates. Utility of AF-648 as a calpain substrate was compared with that of suc-LLVY-AMC by endpoint measurement. The rate of increase in fluorescence of AF-648 in the presence of C1 was 12.7-fold lower than that of suc-LLVY-AMC. Both suc-LLVY-AMC and AF-648 were cleaved more efficiently by C1 than by C2 (1.2-fold and 1.7-fold, respectively). (C) C1 after 10 min of autolysis. Preincubation of C1 to induce autolysis resulted in ∼50% reduction in the level of CAPN1 protein, as assessed by signal intensity. (D) Inhibitory effect of C2 on C1. Autolyzed C1 (C1auto) exhibited a ∼65% decrease in protease activity in the assay using AF-648 (black). When incubation was performed in the presence of C2, an additional ∼40% decrease in the activity toward AF-648 was observed (stripe). Under the same conditions, the protease-inactive mutant C2:CS did not affect the activity of C1. C1 and C2 were mixed at a molar and activity ratio of 40:1 and 30:1, respectively. Activity was measured using the initial velocity of the reaction; *, P<0.05; **, P<0.01; ***, P<0.001.
