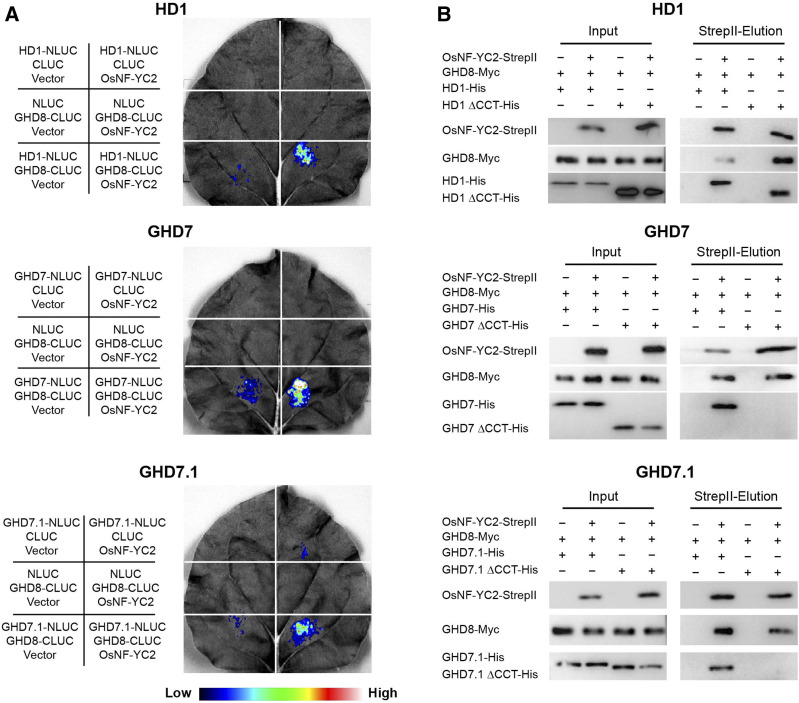
Figure 1.
CCT Domain-Containing Proteins from All Three Families Can Be Physically Associated with the GHD8/OsNF-YC2 Dimer.
(A) LCI assay shows the interactions among the full-length CCT domain-containing proteins, GHD8, and OsNF-YC2. For each representation, the constructs used for each infiltration were listed at the left corresponding section. Fluorescence intensities represented their interaction activities. CCT domain-containing proteins (HD1, GHD7, and GHD7.1) and GHD8 were fused to the N- and C-terminal domains of LUCIFERASE (NLUC and CLUC), respectively. OsNF-YC2 was cloned into the third vector pCAMBIA1301s. Co-transfections of CCT-NLUC (HD1, GHD7, or GHD7.1), GHD8-CLUC, and OsNF-YC2 could produce robust luciferase activities, while other infiltrations lacking one or two proteins brought about no or slight signals.
(B) Pull-down assay showed the interactions among the full-length CCT domain-containing proteins, GHD8, and OsNF-YC2. His-tagged CCT protein (HD1, GHD7, or GHD7.1), Myc-tagged GHD8, and StrepII-tagged OsNF-YC2 were co-transfected into Expi293F cells. The supernatant from lysed cells was loaded onto StrepII beads. Both GHD8 and CCT proteins were co-eluted with OsNF-YC2. The proteins were detected by immunoblot with antibodies against StrepII, His, and Myc. ΔCCT indicated deletion of CCT domain.