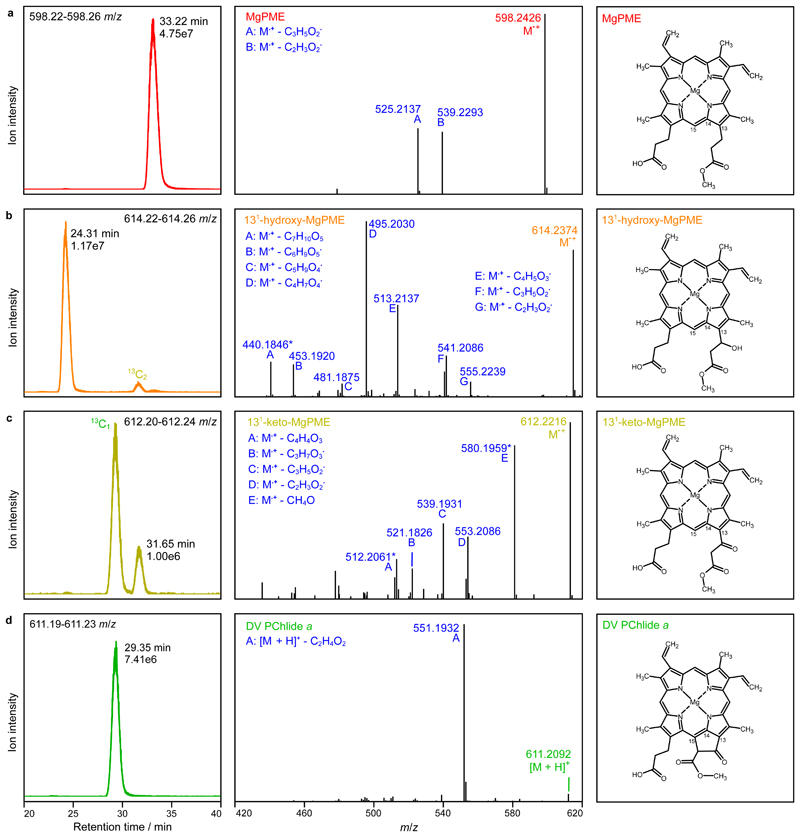
Fig. 5. Analysis of extracted pigments by LC-ESI-MS/MS.
The pigment extract from scaled-up in vitro cyclase assays corresponding to 4x AcsF (0.92 µM) in Fig. 4 was analysed. Extracted ion chromatograms (EICs) and product ion spectra derived from HCD of selected monoisotopic molecular ions are shown in the left and centre panels, respectively: a, MgPME, b, 131-hydroxy-MgPME, c, 131-keto-MgPME, d, DV PChlide a. The molecular structures that align with the mass spectral evidence presented here are shown in the corresponding right panels. EICs were generated for the indicated m/z ranges covering the target monoisotopic ions with peaks labelled with their retention times and ion intensities. Peaks mapping to 13C-containing isotopomers that fall within the EIC range are also labelled. Cations generated by gas phase neutral loss reactions are indicated by upper case letters with the eliminated molecular formulas also listed. The majority of product ions are carbocations formed after radical neutral loss; those labelled with an asterisk are radical cations formed after even electron neutral loss. Details of the structures which validate the identifications of the cyclase substrate, intermediates and product are shown in Supplementary Fig. 3.