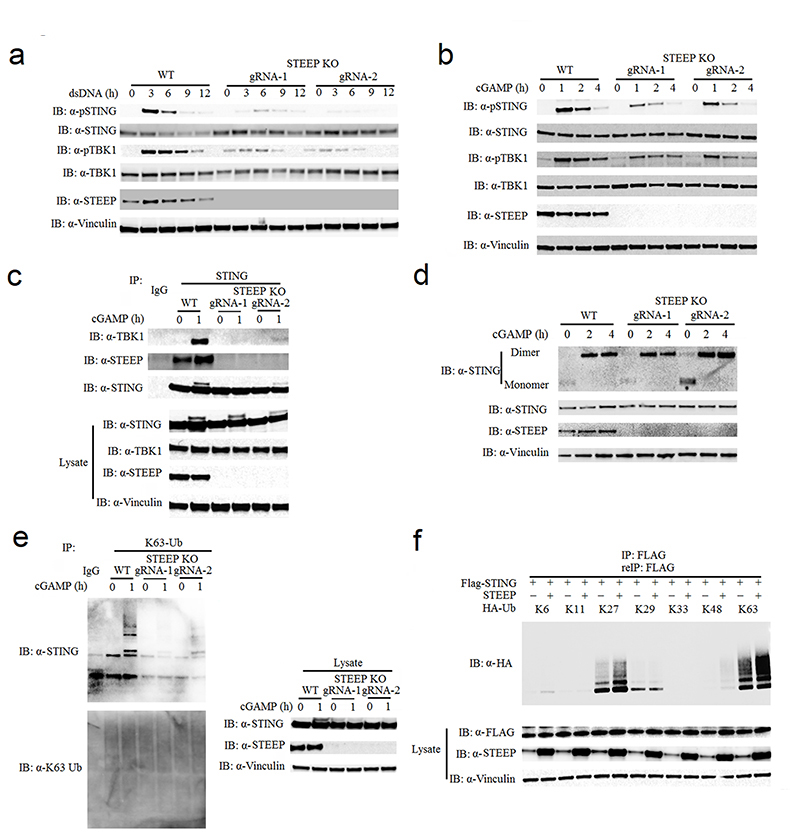
Figure 3. STEEP is essential for STING ubiquitination and recruitment of TBK1.
(a, b) Immunoblot analysis for the indicated proteins from whole cell lysates of WT or STEEP-deficient THP-1 cells after stimulation with (a) dsDNA or (b) cGAMP for the indicated time intervals. (n = 3 biologically independent experiments). (c) Immunoblot analysis of proteins co-immunoprecipitated with endogenous STING probed with the indicated antibodies. The lysates used for the immunoprecipitation were from WT or STEEP-deficient THP-1 cells stimulated with cGAMP for 1 h. (n = 3 biologically independent experiments). (d) STING dimerization assay. Immunoblot analysis with anti-STING probing of lysates from WT or STEEP-deficient THP-1 cells after stimulation with cGAMP for the indicated time intervals. The lysates were run on non-reducing SDS-PAGE prior to blotting. For controls reduced samples were run in parallel and blotted for STING, STEEP, and vinculin. (n = 3 biologically independent experiments). (e) K63-linkage ubiquitin TUBE assay and immunoblotting using anti K63-linkage ubiquitin and anti-STING antibodies. The material used was whole cell lysates from WT or STEEP-deficient THP-1 cells stimulated with cGAMP for 1 h. (n = 3 biologically independent experiments). (f) HEK-293T cells were transfected with FLAG-tagged STING, STEEP, HA-tagged Ub-K11, Ub-K27, Ub-K29, Ub-K33, Ub-K48 or Ub-K63 before co-immunoprecipitation and immunoblot analysis were performed with the indicated antibodies. (n = 3 biologically independent experiments).