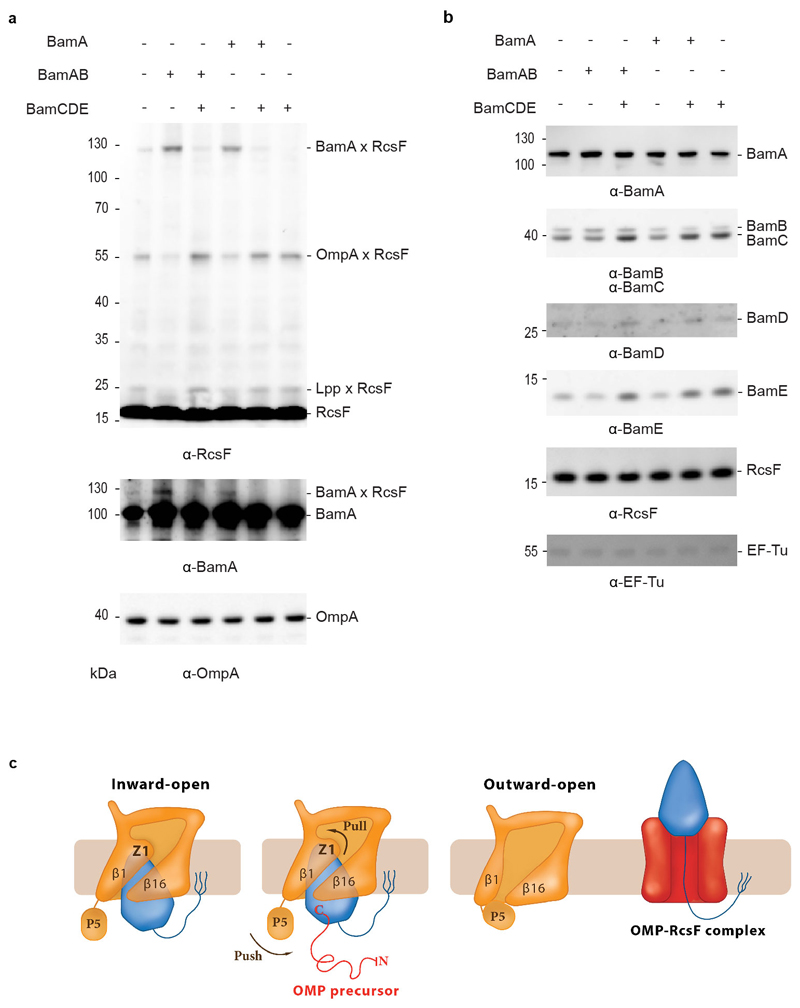
Figure 4. BamA-RcsF is a proxy for the inward-open conformation of BamA.
(a) In vivo chemical crosslinking of RcsF with BamA and OmpA. The BamA-RcsF complex accumulates when either BamA alone or BamAB together are moderately over-expressed from a plasmid in cells also expressing BAM at physiological levels from the chromosome. The copies of BamA and BamAB in excess are not functional (BamCDE is required for BAM activity) and do not funnel RcsF to its OMP partners. As a result, RcsF accumulates on BamA and OmpA-RcsF does not form. Over-expression of BamCDE (also from a plasmid) in these cells restores the stoichiometry between the BAM components: BamA-RcsF does not accumulate and the formation of OmpA-RcsF is restored. As shown previously10, levels of OmpA-RcsF are inversely correlated with BamA-RcsF. Overexpression of the BamCDE sub-complex alone does not impact the activity of the BAM complex expressed from the chromosome: wild-type BamA-RcsF and OmpA-RcsF levels are observed. Wild-type BamA-RcsF and OmpA-RcsF levels are also detected when BamA and BamCDE are overexpressed together, as expected given that BamB is not essential. RcsF also forms a complex with the abundant lipoprotein Lpp (Lpp-RcsF), as in10. Protein expression levels of OmpA were analyzed by immunoblot in the non-crosslinked samples, showing no differences. The additional bands that are detected in the lanes where BamA-RcsF is not observed likely correspond to poorly abundant complexes between RcsF and unknown proteins. n= 3 biologically independent experiments. (b) Protein expression levels of BamB, BamC, BamD, BamE and RcsF from no-crosslinked samples overexpressing BamA (pBamA), BamAB (pBamA-B) and BamCDE (pSC263) were analyzed by western blot. EF-Tu expression levels were analyzed as loading control. n= 3 biologically independent experiments. (c) Model proposing that BamA conformational cycling is triggered by incoming OMP substrates on the BAM holocomplex. A BamA inward-to-outward open transition could result in an upward displacement of RcsF via a push-and-pull mechanism, resulting in an OMP-RcsF complex. The push-and-pull mechanism involves BamA POTRA5 (P5) and Z1. The topology of the OMP-RcsF complex remains to be established. For clarity, POTRA1-4 and the BAM lipoproteins have been ommitted.