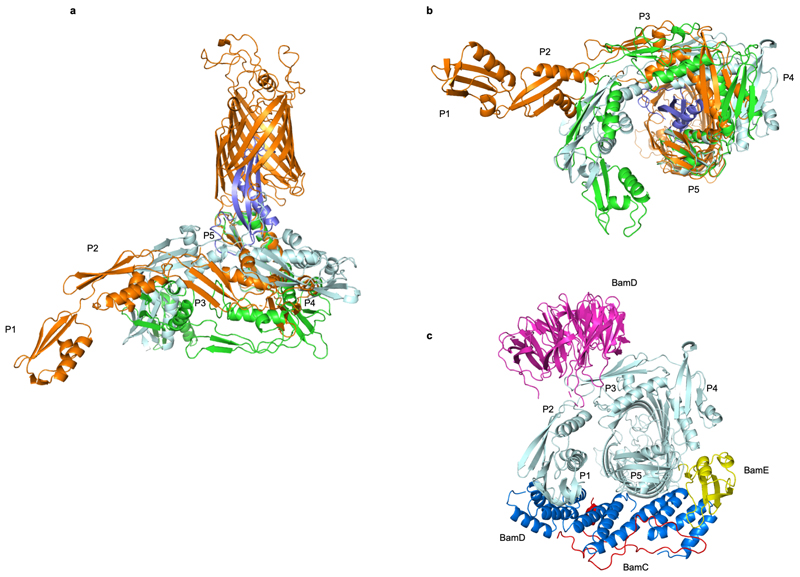
Extended Data Fig. 3. Structural dynamics of the BamA POTRA domains.
(a, b) Superimposition of BamA-RcsF (gold and blue, respectively) with the POTRA domains in the inward-open BamABCDE complex (PDB: 5D0O; light blue) or the outward-open BamACDE complex (PDB: 5EKQ; green). Complexes are superimposed based on 400 equivalent Cα atoms in the BamA β-barrel, and shown in side (a) or periplasmic (b) view. For 5d0o and 5ekq, the accessory Bam subunits and the BamA β-barrel are omitted for clarity. (c) Periplasmic view of the inward-open BamABCDE complex, showing binding of the BAM accessory proteins BamB (magenta), BamC (red), BamD (blue), and BamE (yellow). Pulldown experiments showed that RcsF binds the BamABCDE complex (Fig. 1). In agreement with this observation, structural comparisons reveal that RcsF binding would not result in direct steric clashes with any BAM accessory protein. However, the positions of the POTRA domains in the BamA-RcsF and BamABCDE complexes are markedly different. In the BamA-RcsF complex, POTRA5 makes a 26 outward rotation to accommodate RcsF (see also Fig. 3), and a reorganization in the joint between POTRA domains 3 and 2 results in a more extended conformation of the POTRA “arm” and the projection of POTRA domains 2 and 1 further from the BamA β-barrel, a conformation not previously reported in available BamA structures. In the BamABCDE complex, BamD contacts both POTRA5 and the joint of POTRA domains 1 and 2. In the BamA-RcsF complex, POTRA5 and POTRA domains 2 and 1 are too distant to be bridged by BamD; binding of BamD to BamA-RcsF therefore requires a conformational change in the POTRA arm or the dissociation of BamD at either of these two contact points.