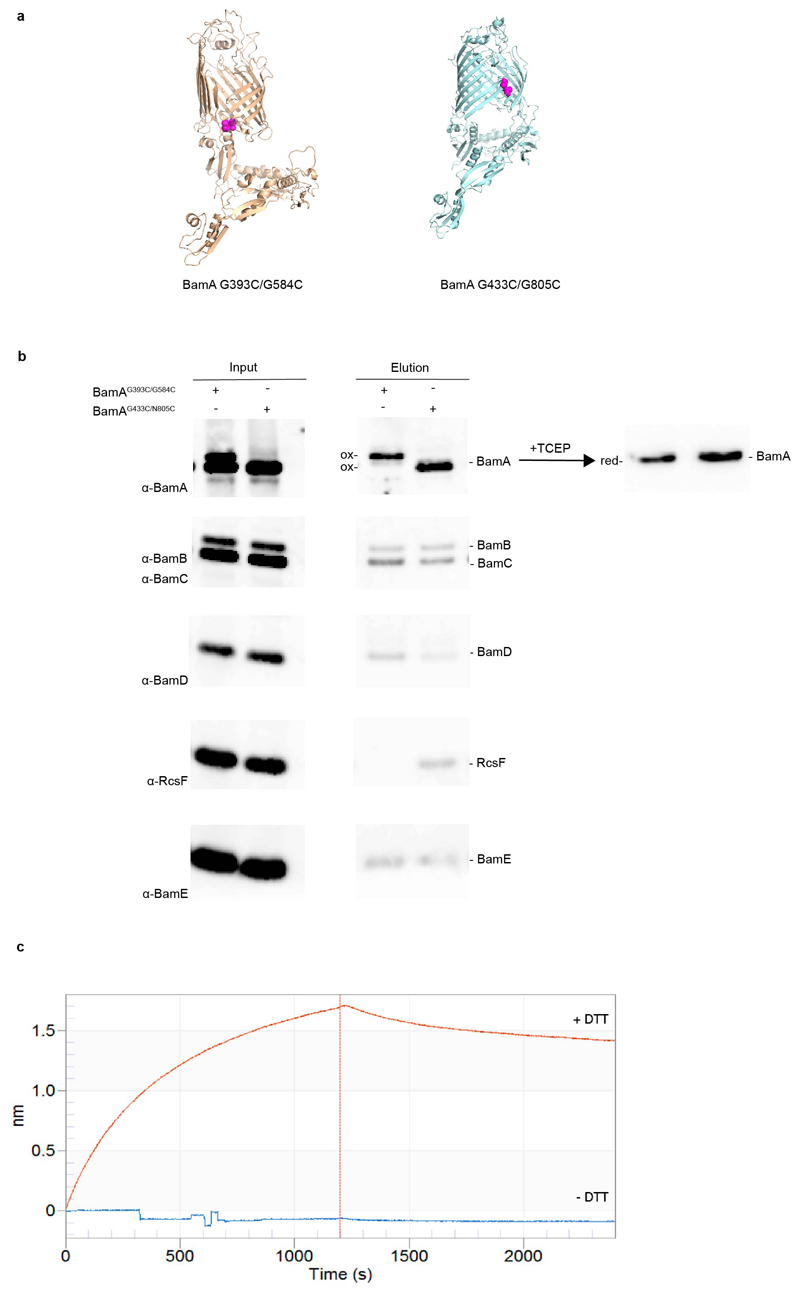
Extended Data Fig. 5. RcsF binds the inward-open conformation of BamA.
Models for the BamAG393C/G584C 5 and BamAG433C/N805C 26 double cysteine mutants, which are locked in the outward-open or inward-open conformation, respectively, when oxidized. Mutated cysteines are shown as atom spheres. (b) BamA barrel locking and RcsF binding. Overexpression of double cysteine mutants pBamAG393C/G584C-B and BamAG433C/N805C-B in a wild-type strain. RcsF can be co-purified with the BamA β-barrel locked in the inward-open conformation (BamAG433C/N805C) by a disulfide bond (ox) but not in the outward-open conformation (BamAG393C/G584C). BamA mutants become reduced (red) following treatment with tris(2-carboxyethyl) phosphine (TCEP) and migrate similarly. The oxidized form of BamAG393C/G584C migrates more slowly than wild-type BamA. As a result, two bands are visible for BamA in the input of BamAG393C/G584C, the lower migrating band corresponding to wild-type BamA expressed from the chromosome. n= 3 biologically independent experiments. (c) Sensorgram from biolayer interferometry of immobilized RcsF titrated with BamAG393C/G584C, without (oxidized; - DTT) or with dithiothreitol (reduced; + DTT). When the β-barrel is locked in the outward-open conformation (- DTT), RcsF is unable to bind BamA. When reduced, BamAG393C/G584C regains binding, demonstrating that BamA reverts to the inward-open conformation in which it can bind RcsF.