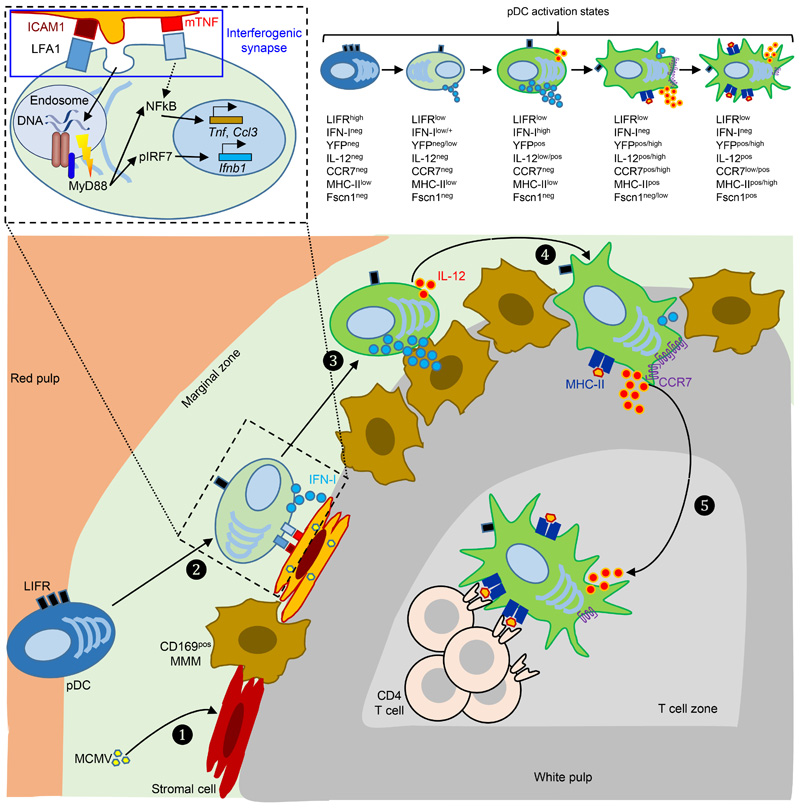
Extended Data Fig. 8. Proposed model of the spatiotemporal dynamics of splenic pDC activation and functions during MCMV infection.
MCMV initially targets and replicates in stromal cells and/or metallophilic macrophages in the marginal zone (❶). These infected cells may then upregulate their expression of ICAM-1 and express mTNF, leading to their specific recognition by, and interactions with, quiescent pDCs. This interaction is proposed to downregulate LIFR expression on pDCs, to induce low levels of Tnf and Ccl3 in pDCs. It may also lead to the generation of an interferogenic synapse (❷) promoting local targeted delivery of viral material from the infected cell to pDCs, as illustrated on the upper left detailed drawing enlarged from the corresponding delimited area in the main drawing. This viral material is engulfed in pDCs and routed into dedicated endosomes, allowing TLR9 triggering, with the downstream enforcement of Tnf and Ccl3 expression and the induction of IFN-I genes. At this early activation state, pDCs from Ifnb1Eyfp reporter mice already start to express IFN-I but not yet clearly detectable levels of YFP. Then, pDCs further enhance their expression of IFN-I, leading to their expression of high levels of YFP in Ifnb1Eyfp reporter mice, and they simultaneously start to express IL-12 (❸). After termination of their IFN-I production, pDCs further enhance their IL-12 production, acquire CCR7 expression and migrate from the marginal zone to the white pulp through bridging channels (❹). Ultimately, pDCs relocate to the T cell zone where they harbor clear features of mature DCs, with a transcriptional, morphologic and functional convergence with tDCs, presumably including the acquisition of a dendritic morphology upon expression of Fscn1 and other genes involved into cytoskeleton remodeling, and acquisition of the ability to prime naïve CD4+ T cells (❺). CD169+ MMM, marginal zone metallophilic macrophages; mTNF, plasma membrane-bound TNF; pIRF7, phosphorylated IRF7.