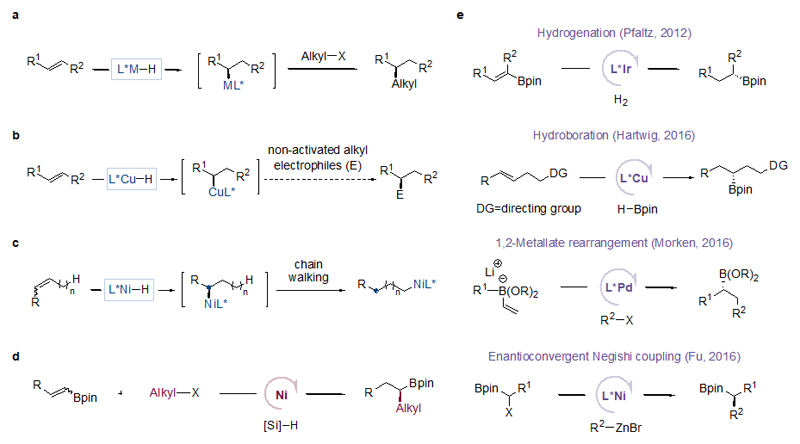
Figure 1. Strategies for enantioselective C(sp3)-C(sp3) cross-coupling.
a, Enantioselective metal hydride insertion to internal olefins followed by enantiospecific alkyl-alkyl coupling. b, Challenge for Cu–H chemistry: no precedent of cross coupling of non-activated alkyl electrophiles with a transient chiral alkyl–Cu intermediate. c, Challenge for Ni–H chemistry: an unwanted chain walking event results in an ablation of the newly generated stereocenter (initially a new stereocenter is created upon metal hydride insertion into an olefin, but the stereocenter is lost due to chain walking generating a primary alkyl–Ni species). d, This work: Ni-catalysed enantioselective cross-coupling of non-activated alkyl halides with internal olefins. e, Comparison to other catalytic methods for the synthesis of chiral alkyl boronates: asymmetric hydrogenation of specialized and hard-to-access substrates by Ir-catalysis, asymmetric hydroboration of olefins bearing a specific directing group by Cu-catalysis, Pd-catalysed asymmetric 1,2-metallate rearrangement followed by cross coupling which employs highly reactive and difficult to handle organolithium reagents (RLi), and Ni-catalysed enantioconvergent cross coupling of α-halo boronates with highly reactive organozinc reagents (RZnBr). These methods provide limited substrate scope and were less practical or general than the method described in the current work. DG = directing group