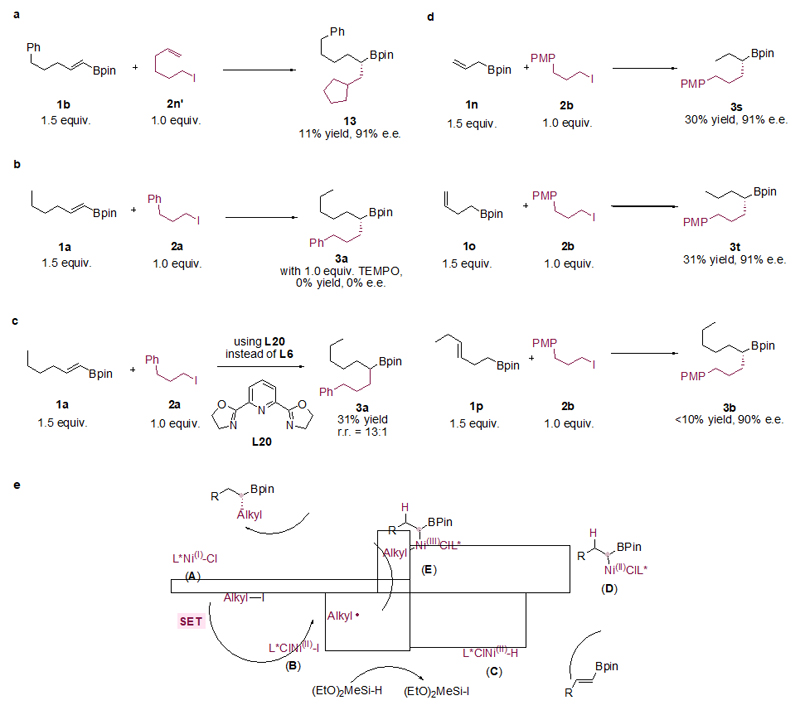
Figure 3. Mechanistic studies of the catalytic enantioselective C(sp3)–C(sp3) cross-coupling.
a, Radical clock experiment with an alkene-tethered alkyl iodide 2n’ afforded the formation of 13 under standard conditions, suggesting the intermediacy of an alkyl radical and its subsequent cyclization and C(sp3)–C(sp3) coupling (see Supplementary Information section 12.4 for full details). b, Reaction was completely shut down by the addition of a radical scavanger TEMPO (see Supplementary Information section 12.5 for full details). c, Probe for origin of regioselectivity by using a tridentate Py-box ligand (L20) instead of L6 (see Supplementary Information section 12.6 for full details). d, Reactions with substrates where the alkenyl group is distal to the Bpin group: chain-walking products were formed with high enantioselectivity under standard conditions (see Supplementary Information section 13 for full details). e, Outline of a possible reaction pathway for Ni-catalysed enantioselective C(sp3)–C(sp3) cross coupling. r.r = Regioisomeric ratio; PMP = paramethoxyphenyl.