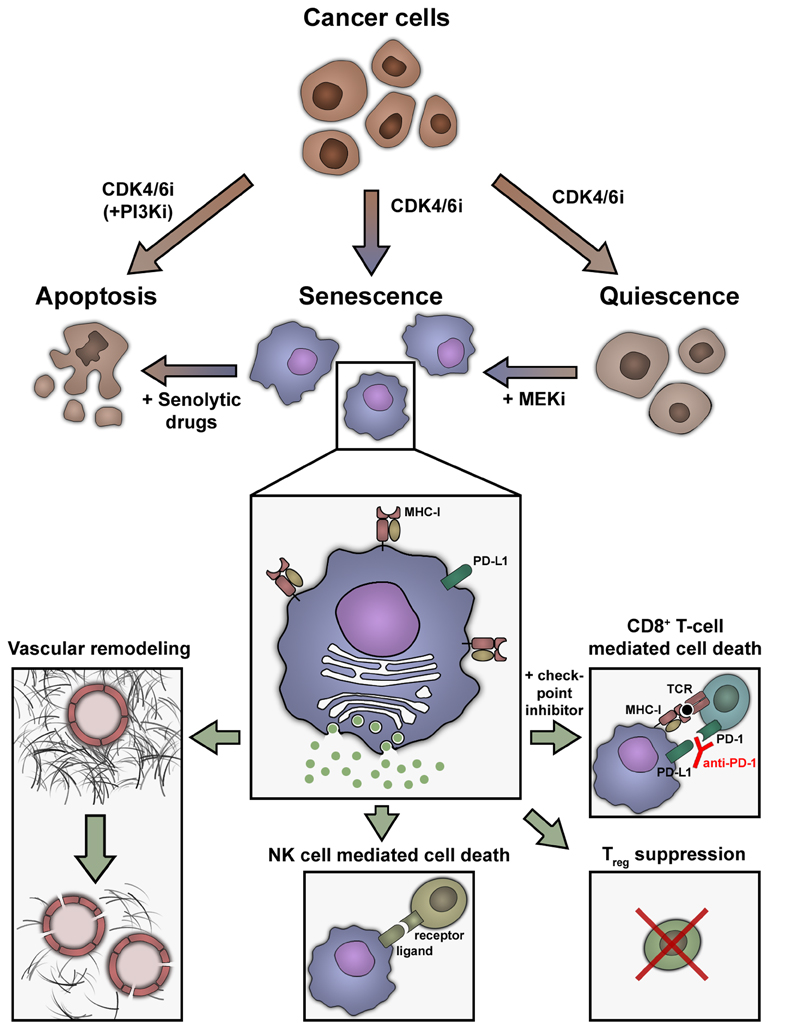
Figure 2. Different cell fates caused by CDK4/6 inhibitors influence treatment outcome.
In cancer cells with a functional RB pathway, treatment with CDK4/6 inhibitors usually induces quiescence or senescence. In some tumor types, senescence can be enforced by combined treatment with MEK inhibitors. The combination of a CDK4/6 inhibitor with a PI3K inhibitor can change the cell fate towards apoptosis, which is rarely seen in a monotherapy setting. Senescent cells have a characteristic phenotype that includes an enlarged and flattened morphology, increased senescence-associated β-Galactosidase activity (“blue cells”) and the senescence-associated secretory phenotype (SASP). The SASP promotes changes in the tumor microenvironment, like vascular remodeling (allowing for increased uptake of chemotherapy in pancreatic cancer) or recruitment of cytotoxic T cells. CDK4/6 inhibitors also induce enhanced expression of MHC-I complexes on cancer cells, facilitating activation of cytotoxic T cells. Combination treatment with checkpoint inhibitors (e.g. an anti-PD-1 antibody) can further improve the immune-mediated clearance of senescent cancer cells. In some tumors, treatment with CDK4/6 inhibitors can also reduce levels of immunosuppressive regulatory T cells or attract NK cells and promote NK-cell mediated cancer cell death.