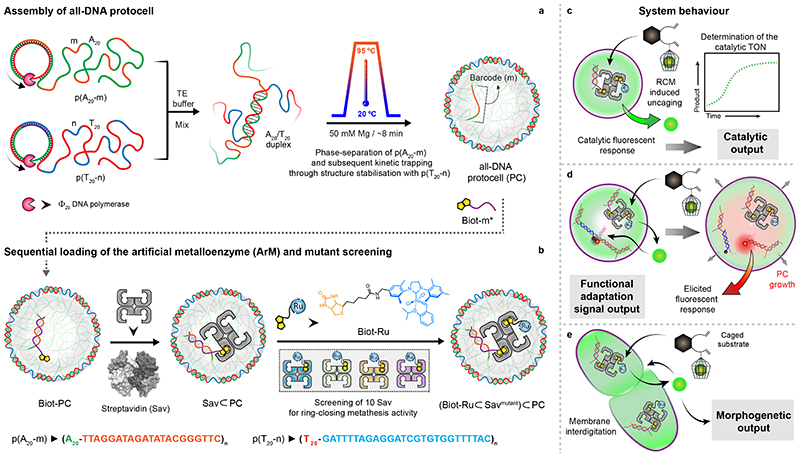
Fig. 1. Design concept, strategy, and system behaviour of artificial metalloenzyme (ArM)-catalysed signal conversion and downstream adaptation inside all-DNA protocells (PCs).
(a) Synthesis of sequence-controlled multiblock ssDNA polymers via rolling circle amplification (RCA). The mixture of the ssDNA polymers is subjected to a heating ramp (3 °C/min) in the presence of Mg2+. Thermally-induced phase-separation of p(A20-m) during heating and duplex (A20/T20) formation (at the coacervate surface) during cooling, leads to the self-assembly of kinetically-trapped all-DNA PCs. The core of the PCs is composed of liquid p(A20-m) while the shell is constituted with p(T20-n) crosslinked via A20/T20 duplexes. The p(A20-m) dissolves below its cloud point temperature, but remains entrapped in a liquid state under high osmotic pressure and macromolecular crowding in the tight hydrogel-like shell. In addition to their straightforward assembly, such PCs allow selective functionalization of the core and the shell using the barcodes (m,n).(b) Sequential loading and assembly of the artificial metalloenzyme (ArM) is achieved by attaching Biot-m* to the core barcode m, followed by addition of streptavidin (Sav) and a biotinylated olefin metathesis catalyst (Biot-Ru), to afford a PC-loaded ArM ((Biot-Ru⊂Savmutant)⊂PC, or ArM⊂PC hereafter). The catalytic activity of the ArM⊂PC was optimized by screening 10 Sav mutants. (c-e) Schematic representation of three adaptive responses of the RCM inside the PCs. (c) The immobilized ArM catalyses a DNA-orthogonal uncaging reaction giving rise to a primary fluorescent signal. (d) The uncaged product instigates swelling of the PCs and destabilizes a mechanofluorescent force module (installed in PCs) which, triggers a secondary fluorescence output. (e) The accumulated uncaged product also induces membrane dynamization of the PCs, resulting in pronounced morphological transformations, ultimately leading to PC fusion.