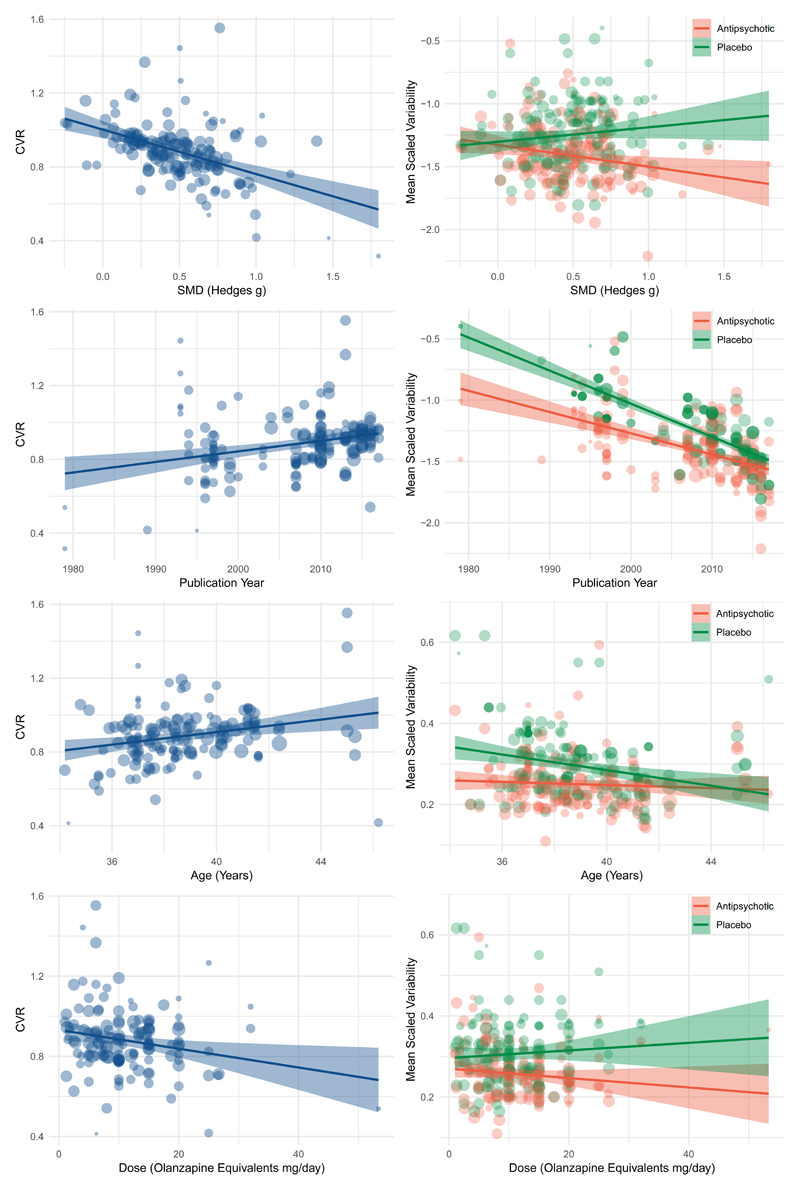
Figure 4.
Figures to the left illustrate meta-regressions exploring the influence of trial characteristics on the coefficient of variation ration (CVR) for change in total symptoms. Figures to the right study the same trial characteristics but examine placebo and antipsychotic arms separately.
(A) Trials that show greater antipsychotic efficacy (greater mean symptom improvement with antipsychotic relative to placebo) show lower variability in antipsychotic treatment response relative to placebo (z=-5.7, p<0.001)
(B) Trials showing greater efficacy show reduced variability in antipsychotic treated arms (z=-2.7, p=0.01), but no significant relationship is apparent with regard to placebo variability (z=1.0, p=0.3).
(C) Older trials show lower variability in antipsychotic treatment response relative to placebo (z=4.0, p<0.001)
(D) Both placebo (z=-11.6, p<0.001) and antipsychotic (z=-7.7, p<0.001) arms show reduced variability in more recent trials.
(E) Trials with younger participants show lower variability in antipsychotic treatment response relative to placebo (z=3.1, p=0.002)
(F) Younger patients show greater variability in the placebo arm(z=2.79, p=0.005), while there is minimal effect on the antipsychotic arm(z=0.0, p=0.7).
(G) Trials using higher antipsychotic doses show lower variability in antipsychotic treatment response relative to placebo (z=-2.62, p=0.009)
(H) Neither antipsychotic (z=-0.4, p=0.68) nor placebo arms (z=0.79, p=0.43) independently show a significant relationship with dose.