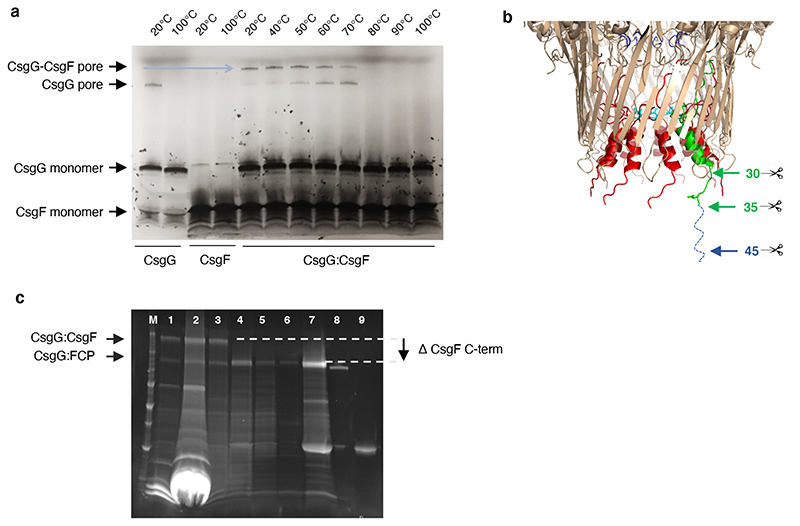
Extended Data Fig. 2. Production and thermal stability of CsgG:CsgF and CsgG:FCP pores.
(a) Production and temperature stability assessment of the CsgG:CsgF pore complex. Incubation of purified CsgG and CsgF in a 1:1 ratio results in the formation of a SDS stable CsgG:CsgF pore complex that is heat stable up to 70 °C. (b) The N-terminal residues of CsgF insert into the CsgG channel and form a second region of constriction, whilst the remaining ~100 residues form a cap like head structure (Figure 1e, f). For nanopore sensing purposes, we sought to produce a complex of CsgG with the CsgF constriction peptide (FCP), lacking the neck and head domains. To do so, CsgG was complexed with CsgF mutants modified to insert a TEV cleavage site at position 30, 35 or 45. The reconstituted CsgG:CsgF pore complexes were digested with TEV protease and analysed by SDS-PAGE (c). M: molecular mass marker, Lane 1, 2: Strep-tag affinity purified CsgG:CsgF complex and excess CsgG, Lane 3: isolation of CsgG:CsgF complex by size exclusion chromatography, Lane 4: CsgG:CsgF35-TEV cleaved with TEV protease to generate CsgG:FCP complex, Lane 5: flow through of CsgG:FCP after strep purification, Lane 6: CsgG:FCP heated to 60°C for 10 minutes. Lane 7: Eluted CsgG:FCP complex from strep column, Lane 8: CsgG pore as the control, Lane 9: TEV protease as the control.