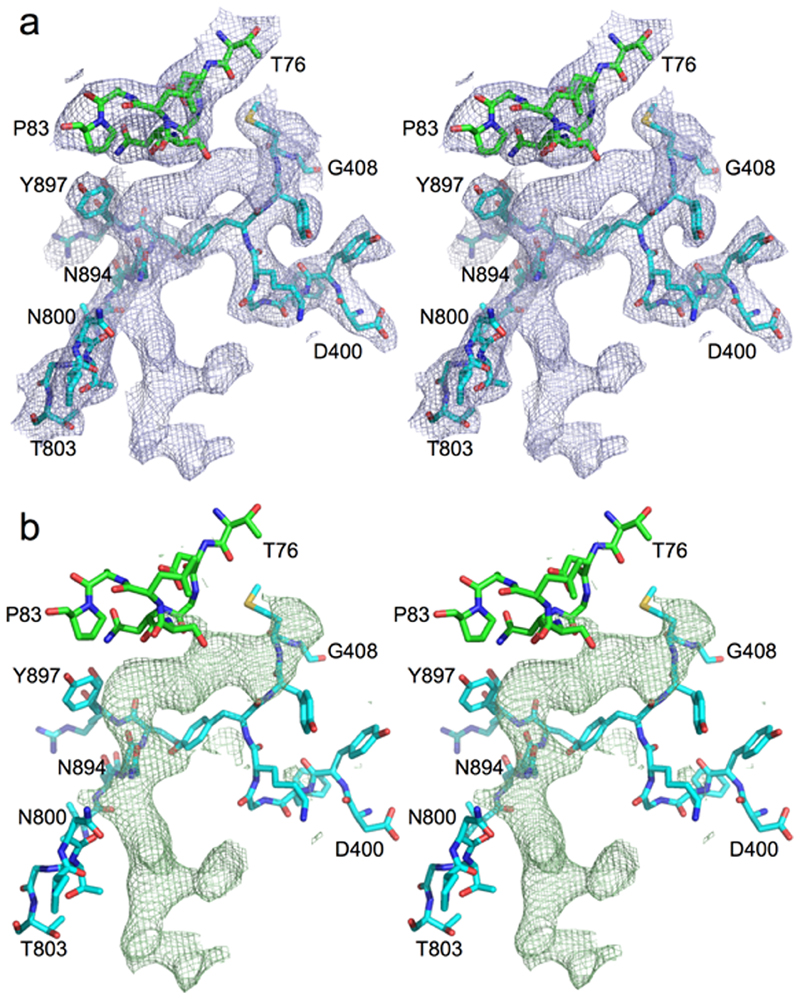
Extended Data Fig. 1. Unbiased peptide density in RagAB.
Stereo diagrams showing 2Fo-Fc (a) and Fo-Fc density (b) in RagAB KRAB before any modelling and refinement of the peptide. Selected segments of RagA (cyan) and RagB (green) neighbouring the peptide are shown. Map contouring parameters are 1.0σ, carve = 2 for the 2Fo-Fc and 3.0σ, carve = 2 for the Fo-Fc map. The extensive contacts of RagA with the peptide are confirmed by a PISA interface analysis17 which shows that 26 RagA residues form an interface with the peptide compared to only 8 for RagB, generating interface areas of 620 and 240 Å2with RagA and RagB respectively. The PISA CSS (complexation significance score) is maximal (1.0) for peptide-RagA while it is only 0.014 for peptide-RagB. This suggests that the observed co-crystal structure represents a state where the ligand has been partially transferred from an initial, presumably low(er)-affinity binding site on RagB to a high(er)-affinity binding site in the RagAB complex, allowing co-purification.