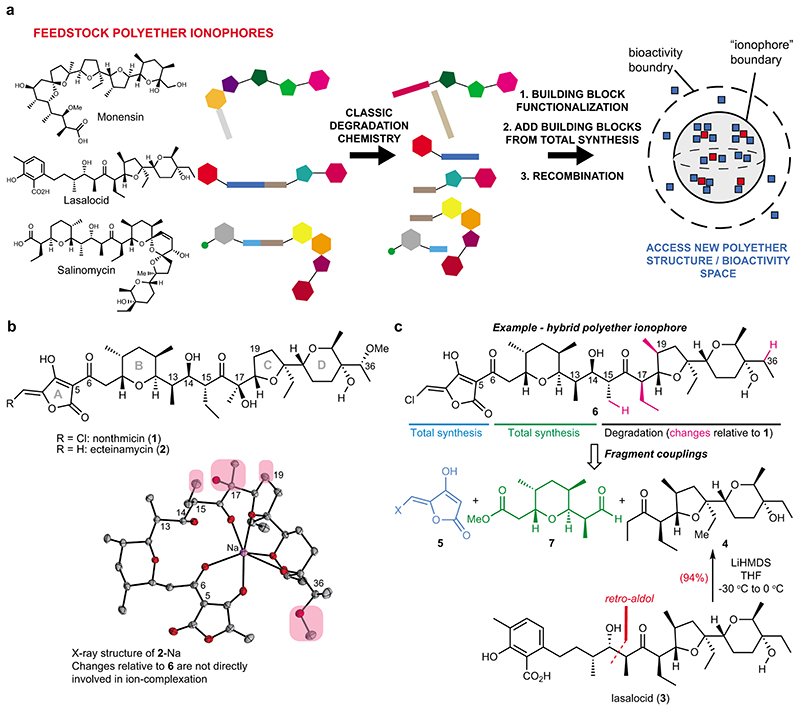
Fig. 1. Accessing structural diversity within the polyether ionophores.
(a) Flowchart depicting the overall concept of reconstructing new polyether scaffolds by recycling elements from abundant feedstock polyether ionophores. The resulting “hybrid” molecules (blue squares) are plotted in a hypothetical structure and bioactivity space to illustrate the relation of these compounds to the natural polyethers (red squares). The compounds that possess ionophore activity constitute a sub-space of a larger bioactivity-space that can be explored using hybrid polyethers. (b) Chemical structures of polyether ionophores nonthmicin and ecteinamycin. Both are active against gram-positive bacteria, with especially strong potency against C. difficile reported for ecteinamycin. The compounds bear resemblance to lysocellin/ferensimycin but the chlorinated methylidene tetronic acid group of nonthmicin is unprecedented. The X-ray structure25 depicts ecteinamycin bound to a single sodium-ion and the chemical groups on the hydrophobic periphery that have been altered in the target hybrid polyether 6 have been circled in pink. No crystal structure of nonthmicin is available. (c) Chemical structure of the hybrid polyether 6 with indication of the required fragments and the origin of these fragments. The main fragment, ketone 4, can be obtained in a single synthetic step from lasalocid.