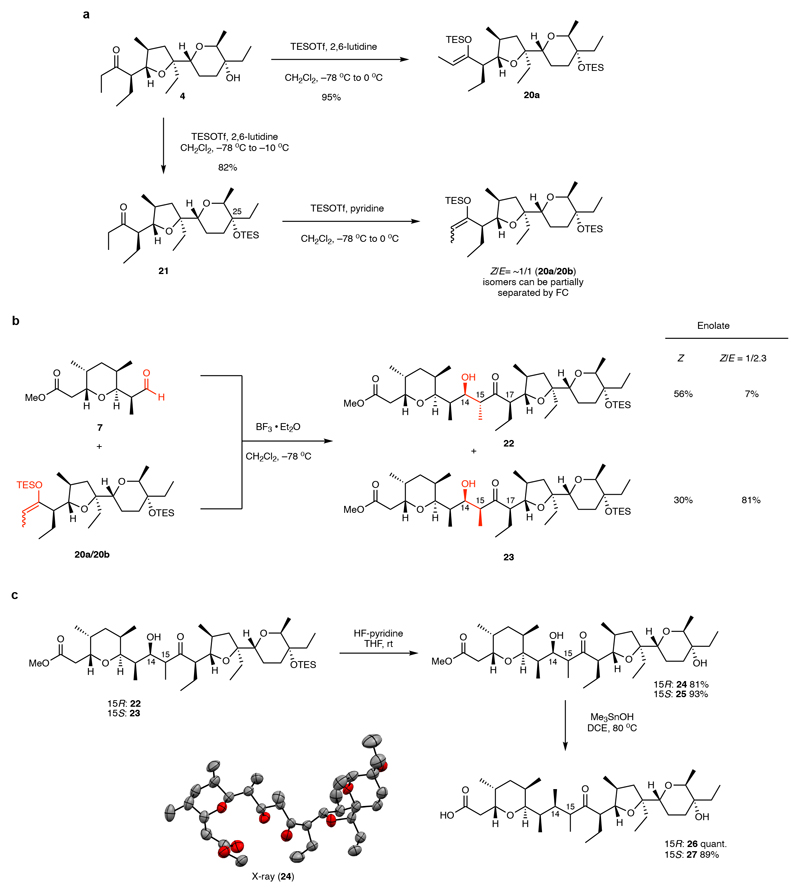
Fig. 3. Fragment-coupling via boron trifluoride-mediated Mukaiyama-aldol reaction.
(a) The (Z)-TES-enolate 20a could be readily obtained, but special procedures had to be developed to access mixtures of (E)- and (Z)-TES-enolates. Purification could be used to further enrich the (E)-TES-enolate 20b. (b) Aldol reaction affords two major products (22 and 23) depending on the configuration of the silyl-enolate derived from ketone 4. Compound 22 was confirmed by X-ray analysis of derivative 24 to be the initially targeted aldol-product (c) Both aldol products 22 and 23 could be processed towards the final fragment coupling in two high-yielding steps. TESOTf = triethylsilyltrifluoromethanesulfonate, THF = tetrahydrofuran, rt = room temperature, DCE = 1,2-dichloroethane.