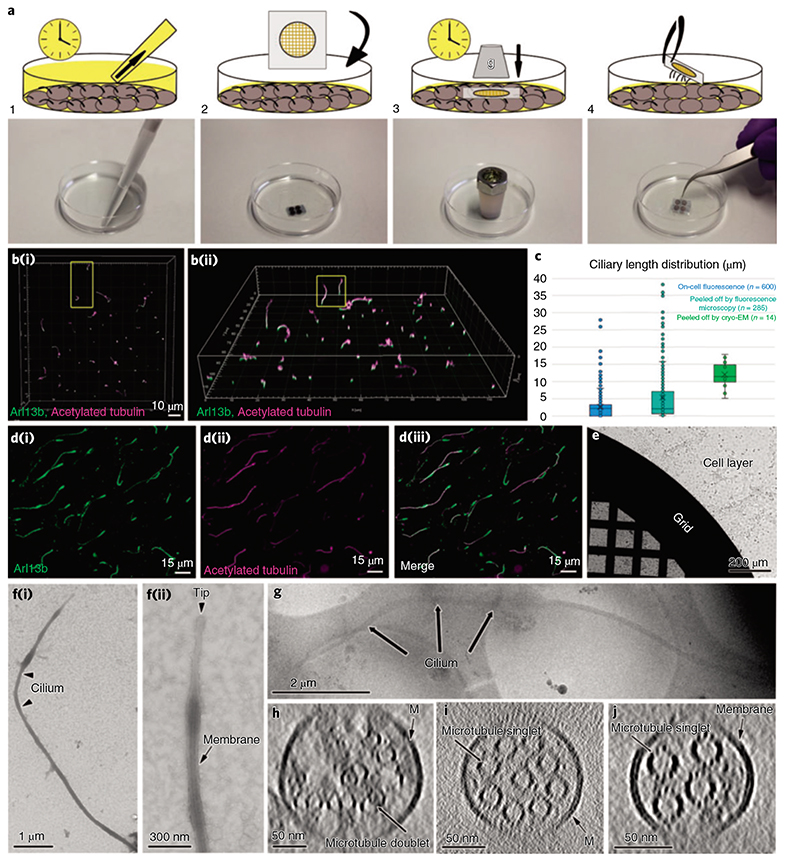
Fig. 2. I Cryo-peel off: a method to prepare primary cilia for cryo-ET.
a, Description of the steps followed for the peel off of primary cilia from MDCK-II monolayers. Step 1: removal of the deciliation buffer from the cell culture; step 2: application of poly-L-lysine-coated EM grids, supported by a glass slide, on the apical surface of the cell layer; step 3: pressure was applied over time on the glass slide to favor the adhesion of cilia to the EM grid; step 4: retrieval of the glass slide/EM grid from the cells’ apical surface and consequent primary cilia peel off. b, Laser scanning confocal fluorescence microscopy of the MDCK-II monolayer showing the variability of ciliary lengths after 2d of cell starvation; (b(i)), top view; (b(ii)), tilted view. c, Distribution of lengths of cilia associated with cells (blue), cilia peeled-off by fluorescence microscopy (cyan) and cilia peeled-off by cryo-EM (green). d, Immunofluorescence staining of peeled-off cilia on a glass slide, showing the colocalization of ciliary membrane (d(i)) and microtubules (d(ii) and the combined images (d(iii)).e, A Quantifoil grid on a cell monolayer during the peel off procedure as depicted in a (steps 2 and 3). f(i), Negative staining TEM of a peeled-off primary cilium; f(ii), the zoomed-in view of the ciliary tip from the same cilium shows the preservation of the ciliary membrane. g, Low-magnification cryo-EM image of a peeled-off and plunge-frozen cilium. h-J, Representative proximodistal cryo-tomographic slices of plunge-frozen cilia (h) close to the base central shaft (i) and distal segment (J).